RSDB: A rare skin disease database to link drugs with potential drug targets for rare skin diseases
- PMID: 36028515
- PMCID: PMC9418253
- DOI: 10.1038/s41597-022-01654-2
RSDB: A rare skin disease database to link drugs with potential drug targets for rare skin diseases
Abstract
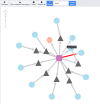
Rare skin diseases include more than 800 diseases affecting more than 6.8 million patients worldwide. However, only 100 drugs have been developed for treating rare skin diseases in the past 38 years. To investigate potential treatments through drug repurposing for rare skin diseases, it is necessary to have a well-organized database to link all known disease causes, mechanisms, and related information to accelerate the process. Drug repurposing provides less expensive and faster potential options to develop treatments for known diseases. In this work, we designed and constructed a rare skin disease database (RSDB) as a disease-centered information depository to facilitate repurposing drug candidates for rare skin diseases. We collected and integrated associated genes, chemicals, and phenotypes into a network connected by pairwise relationships between different components for rare skin diseases. The RSDB covers 891 rare skin diseases defined by the Orphanet and GARD databases. The organized network for each rare skin disease comprises associated genes, phenotypes, and chemicals with the corresponding connections. The RSDB is available at https://rsdb.cmdm.tw .
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Simone Baldovino M, Domenica Taruscio M, Dario Roccatello M. Rare diseases in Europe: from a wide to a local perspective. Sat. 2016;12:20. - PubMed
Publication types
MeSH terms
Grants and funding
- MOST109-2823-8-002-010-CV, MOST 109-2320-B-002-040-, MOST 110-2320-B-002-038-/Ministry of Science and Technology, Taiwan (Ministry of Science and Technology of Taiwan)
- MOHW110-FDA-D-114-000611/Ministry of Health and Welfare, Taiwan | Food and Drug Administration (Taiwan Food and Drug Administration)
- NTU-CC-110L890803, NTU-110L8809/National Taiwan University (NTU)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

