A biomechanical-based approach to scale blast-induced molecular changes in the brain
- PMID: 36028539
- PMCID: PMC9418170
- DOI: 10.1038/s41598-022-17967-6
A biomechanical-based approach to scale blast-induced molecular changes in the brain
Abstract
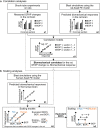
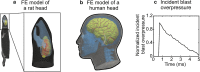
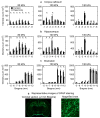
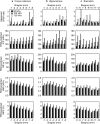
Animal studies provide valuable insights on how the interaction of blast waves with the head may injure the brain. However, there is no acceptable methodology to scale the findings from animals to humans. Here, we propose an experimental/computational approach to project observed blast-induced molecular changes in the rat brain to the human brain. Using a shock tube, we exposed rats to a range of blast overpressures (BOPs) and used a high-fidelity computational model of a rat head to correlate predicted biomechanical responses with measured changes in glial fibrillary acidic protein (GFAP) in rat brain tissues. Our analyses revealed correlates between model-predicted strain rate and measured GFAP changes in three brain regions. Using these correlates and a high-fidelity computational model of a human head, we determined the equivalent BOPs in rats and in humans that induced similar strain rates across the two species. We used the equivalent BOPs to project the measured GFAP changes in the rat brain to the human. Our results suggest that, relative to the rat, the human requires an exposure to a blast wave of a higher magnitude to elicit similar brain-tissue responses. Our proposed methodology could assist in the development of safety guidelines for blast exposure.
© 2022. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

