A circuit from lateral septum neurotensin neurons to tuberal nucleus controls hedonic feeding
- PMID: 36028570
- PMCID: PMC9763109
- DOI: 10.1038/s41380-022-01742-0
A circuit from lateral septum neurotensin neurons to tuberal nucleus controls hedonic feeding
Abstract
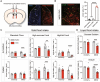
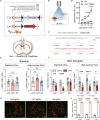
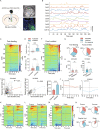
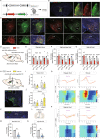
Feeding behavior is regulated by both the homeostatic needs of the body and hedonic values of the food. Easy access to palatable energy-dense foods and the consequent obesity epidemic stress the urgent need for a better understanding of neural circuits that regulate hedonic feeding. Here, we report that neurotensin-positive neurons in the lateral septum (LSNts) play a crucial role in regulating hedonic feeding. Silencing LSNts specifically promotes feeding of palatable food, whereas activation of LSNts suppresses overall feeding. LSNts neurons project to the tuberal nucleus (TU) via GABA signaling to regulate hedonic feeding, while the neurotensin signal from LSNts→the supramammillary nucleus (SUM) is sufficient to suppress overall feeding. In vivo calcium imaging and optogenetic manipulation reveal two populations of LSNts neurons that are activated and inhibited during feeding, which contribute to food seeking and consumption, respectively. Chronic activation of LSNts or LSNts→TU is sufficient to reduce high-fat diet-induced obesity. Our findings suggest that LSNts→TU is a key pathway in regulating hedonic feeding.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–67. doi: 10.1016/S0140-6736(10)62037-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

