The current state of the art and future trends in RAS-targeted cancer therapies
- PMID: 36028717
- PMCID: PMC9412785
- DOI: 10.1038/s41571-022-00671-9
The current state of the art and future trends in RAS-targeted cancer therapies
Abstract
Despite being the most frequently altered oncogenic protein in solid tumours, KRAS has historically been considered 'undruggable' owing to a lack of pharmacologically targetable pockets within the mutant isoforms. However, improvements in drug design have culminated in the development of inhibitors that are selective for mutant KRAS in its active or inactive state. Some of these inhibitors have proven efficacy in patients with KRASG12C-mutant cancers and have become practice changing. The excitement associated with these advances has been tempered by drug resistance, which limits the depth and/or duration of responses to these agents. Improvements in our understanding of RAS signalling in cancer cells and in the tumour microenvironment suggest the potential for several novel combination therapies, which are now being explored in clinical trials. Herein, we provide an overview of the RAS pathway and review the development and current status of therapeutic strategies for targeting oncogenic RAS, as well as their potential to improve outcomes in patients with RAS-mutant malignancies. We then discuss challenges presented by resistance mechanisms and strategies by which they could potentially be overcome.
© 2022. Springer Nature Limited.
Conflict of interest statement
V.V. has received fees for consulting or serving on advisory boards from Amgen, AstraZeneca, Bristol Myers Squibb (BMS), EMD Serono, Foundation Medicine, GlaxoSmithKline, Iteos Therapeutics, Merck, Novartis and Novocure. B.G.N. is a co-founder, holds equity in and receives consulting fees from Lighthorse Therapeutics and Navire Pharmaceuticals; is a co-founder and holds equity in Northern Biologics; is a scientific advisory board (SAB) member for, holds equity in and receives consulting fees from Arvinas; and is an SAB member for and holds equity in Recursion Pharma. K.-K.W. is a co-founder of and holds equity in G1 Therapeutics; has received grants from Alkermes, Ansun, AstraZeneca, BMS, Dracen, Merus, Mirati, Takeda and Tvardi; grants and personal fees from Delfi, Janssen, Pfizer and Zentalis; personal fees from Navire, Prelude and Recursion; and grants from Ono outside the submitted work. S.R.P. declares no competing interests.
Figures


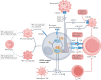
References
-
- Barbacid M. ras genes. Annu. Rev. Biochem. 1987;56:779–827. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

