Local immune cell contributions to fracture healing in aged individuals - A novel role for interleukin 22
- PMID: 36028760
- PMCID: PMC9440089
- DOI: 10.1038/s12276-022-00834-9
Local immune cell contributions to fracture healing in aged individuals - A novel role for interleukin 22
Abstract
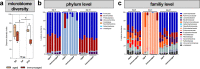
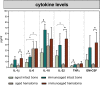
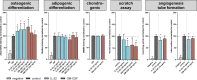
With increasing age, the risk of bone fractures increases while regenerative capacity decreases. This variation in healing potential appears to be linked to adaptive immunity, but the underlying mechanism is still unknown. This study sheds light on immunoaging/inflammaging, which impacts regenerative processes in aging individuals. In an aged preclinical model system, different levels of immunoaging were analyzed to identify key factors that connect immunoaged/inflammaged conditions with bone formation after long bone fracture. Immunological facets, progenitor cells, the microbiome, and confounders were monitored locally at the injury site and systemically in relation to healing outcomes in 12-month-old mice with distinct individual levels of immunoaging. Bone tissue formation during healing was delayed in the immunoaged group and could be associated with significant changes in cytokine levels. A prolonged and amplified pro-inflammatory reaction was caused by upregulated immune cell activation markers, increased chemokine receptor availability and a lack of inhibitory signaling. In immunoaged mice, interleukin-22 was identified as a core cell signaling protein that played a central role in delayed healing. Therapeutic neutralization of IL-22 reversed this specific immunoaging-related disturbed healing. Immunoaging was found to be an influencing factor of decreased regenerative capacity in aged individuals. Furthermore, a novel therapeutic strategy of neutralizing IL-22 may successfully rejuvenate healing in individuals with advanced immune experiences.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

