Acute dizziness and mental alteration associated with Moderna COVID-19 vaccine: a case report
- PMID: 36028809
- PMCID: PMC9412800
- DOI: 10.1186/s12883-022-02834-8
Acute dizziness and mental alteration associated with Moderna COVID-19 vaccine: a case report
Abstract
Background: Due to a rising number of COVID-19 cases, the Indonesian government implemented public health programs to lower the rate. Since January 2021, one of the government's primary policies has been the COVID-19 immunization program. Recently, the Moderna messenger ribonucleic acid (mRNA) vaccine is one of the COVID-19 vaccines used in Indonesia. Based on some research, Moderna has possible side effects throughout the body, including neurological symptoms.
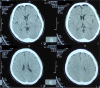
Case presentation: We describe a 39-year-old female with uncontrolled hypertension who showed behavioral change, communication difficulty, social withdrawal, and a confused state within 7 days from getting her first dose of the Moderna vaccine. The patient had a history of febrile convulsion in childhood. An increase of neutrophil-to-lymphocyte ratio (16.9) and C-reactive protein level (31.75 mg/L) indicates ongoing inflammation. Head CT scan shows no abnormalities. She received ceftriaxone, citicoline, and methylprednisolone. The patient was discharged on the seventh day and completely recovered 1 week later. This study is the first case report of encephalopathy following the administration of the Moderna COVID-19 vaccine reported in Indonesia up to our knowledge.
Conclusion: Encephalopathy related to the Moderna COVID-19 vaccine should be acknowledged as an adverse effect of the Moderna COVID-19 vaccine.
Keywords: Acute dizziness; Encephalopathy; Mental alteration; Moderna; Vaccine.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Kadali RAK, Janagama R, Peruru S, Gajula V, Madathala RR, Chennaiahgari N, et al. Non-life-threatening adverse effects with COVID-19 mRNA-1273 vaccine: a randomized, cross-sectional study on healthcare workers with detailed self-reported symptoms. J Med Virol. 2021;93(7):4420–4429. doi: 10.1002/jmv.26996. - DOI - PMC - PubMed
-
- Tam J, Tran D, Bettinger JA, Moore D, Sauvé L, Jadavji T, et al. Review of pediatric encephalitis and encephalopathy cases following immunization reported to the Canadian immunization monitoring program active (IMPACT) from 1992 to 2012. Vaccine. 2020;38(28):4457–4463. doi: 10.1016/j.vaccine.2020.04.035. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials