Disease burden in patients with acute hepatic porphyria: experience from the phase 3 ENVISION study
- PMID: 36028858
- PMCID: PMC9419398
- DOI: 10.1186/s13023-022-02463-x
Disease burden in patients with acute hepatic porphyria: experience from the phase 3 ENVISION study
Abstract
Background: Acute hepatic porphyria (AHP) is a family of four rare genetic diseases, each involving deficiency in a hepatic heme biosynthetic enzyme. Resultant overproduction of the neurotoxic intermediates δ-aminolevulinic acid (ALA) and porphobilinogen (PBG) leads to disabling acute neurovisceral attacks and progressive neuropathy. We evaluated the AHP disease burden in patients aged ≥ 12 years in a post hoc analysis of the Phase 3, randomized, double-blind, placebo-controlled ENVISION trial of givosiran (NCT03338816), an RNA interference (RNAi) therapeutic that targets the enzyme ALAS1 to decrease ALA and PBG production. We analyzed baseline AHP severity via chronic symptoms between attacks, comorbidities, concomitant medications, hemin-associated complications, and quality of life (QOL) and evaluated givosiran (2.5 mg/kg monthly) in patients with and without prior hemin prophylaxis on number and severity of attacks and pain scores during and between attacks.
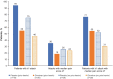
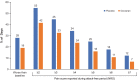
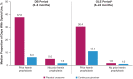
Results: Participants (placebo, n = 46; givosiran, n = 48) included patients with low and high annualized attack rates (AARs; range 0-46). At baseline, patients reported chronic symptoms (52%), including nausea, fatigue, and pain; comorbidities, including neuropathy (38%) and psychiatric disorders (47%); concomitant medications, including chronic opioids (29%); hemin-associated complications (eg, iron overload); and poor QOL (low SF-12 and EuroQol visual analog scale scores). A linear relationship between time since diagnosis and AAR with placebo suggested worsening of disease over time without effective treatment. Givosiran reduced the number and severity of attacks, days with worst pain scores above baseline, and opioid use versus placebo.
Conclusions: Patients with AHP, regardless of annualized attack rates, have considerable disease burden that may partly be alleviated with givosiran.
Keywords: Acute hepatic porphyria; Chronic symptoms; Disease burden; Givosiran; Porphyria attack; Quality of life.
© 2022. The Author(s).
Conflict of interest statement
Bruce Wang reports being a scientific advisor to Alnylam Pharmaceuticals and Recordati Rare Diseases. Paolo Ventura reports receiving advisory board fees and lecture fees from Alnylam Pharmaceuticals and advisory board fees from Recordati Rare Diseases. Kei-ichiro Takase reports having nothing to disclose. Manish Thapar reports being a consultant and speaker for Alnylam. David Cassiman and the University of Leuven, University Hospital Leuven report receiving research grants, travel and conference bursaries, speaker fees, and advisory board compensation from a.o. Sanofi-Genzyme, Takeda-Shire, Alexion, Alnylam, BioMarin, Actelion, Bayer, Roche, BMS, Schering-Plough, Synageva, and Chiesi. Ilja Kubisch reports receiving fees from Alnylam. Shangbin Liu reports being an employee of and owning stock and stock options in Alnylam. Marianne T. Sweetser reports being an employee of and owning stock and stock options in Alnylam. Manisha Balwani reports receiving grant support, consulting fees, advisory board fees, and lecture fees from Alnylam Pharmaceuticals, advisory board fees from Recordati Rare Diseases, grant support and advisory board fees from Mitsubishi Tanabe, and advisory board fees from Alexion, Genzyme/Sanofi, and Takeda. In addition, Mount Sinai faculty are named co-inventors with Alnylam on a patent related to the development of givosiran, the study drug. The Icahn School of Medicine at Mount Sinai receives payments related to this patent from Alnylam, and a portion of these payments are also distributed to faculty and other co-inventors.
Figures





References
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

