Use of dexmedetomidine in patients with sepsis: a systematic review and meta-analysis of randomized-controlled trials
- PMID: 36029410
- PMCID: PMC9420168
- DOI: 10.1186/s13613-022-01052-2
Use of dexmedetomidine in patients with sepsis: a systematic review and meta-analysis of randomized-controlled trials
Abstract
Background: Dexmedetomidine is widely used in patients with sepsis. However, its effect on septic patients remains controversial. The objective of this study was to summarize all randomized controlled trials (RCTs) examining dexmedetomidine use in sepsis patients.
Methods: This systematic review and meta-analysis included RCTs comparing dexmedetomidine with other sedatives in adult sepsis patients. We generated pooled relative risks (RRs) and standardized mean differences and performed trial sequential analysis and a cumulative meta-analysis. The primary outcome was mortality, and the secondary outcomes were the length of the intensive care unit stay, duration of mechanical ventilation, number of ventilation-free days, incidence of total adverse event, incidence of delirium, and levels of interleukin 6, tumor necrosis factor alpha, and alanine aminotransferase.
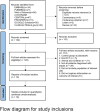
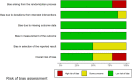
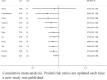
Results: We included 19 RCTs that enrolled 1929 patients. Compared with other sedatives, dexmedetomidine decreased the all-cause mortality (RR 0.83; 95% confidence interval [CI] [0.69, 0.99]) and inflammatory response (interleukin 6 and tumor necrosis factor alpha levels at 24 h: standardized mean difference (SMD) - 2.15; 95% CI [- 3.25, - 1.05] and SMD - 1.07, 95% CI [- 1.92, - 0.22], respectively). Trial sequential analysis showed that it is not up to required information size. The overall risk adverse events was similar between dexmedetomidine and the other sedatives (RR 1.27, 95% CI [0.69, 2.36]), but dexmedetomidine increased the risk of arrhythmias (RR 1.43, 95% CI [0.59, 3.51]). Length of intensive care unit stay (SMD - 0.22; 95% CI [- 0.85, - 0.41]), duration of mechanical ventilation (SMD 0.12; 95% CI [- 1.10, 1.35]), incidence of delirium (RR 0.98; 95% CI [0.72, 1.33]), and levels of alanine aminotransferase and creatinine at 24 h were not significantly reduced.
Conclusions: Dexmedetomidine in sepsis patients could significantly reduce mortality compared with benzodiazepines but not with propofol. In addition, dexmedetomidine can significantly decrease inflammatory response in patients with sepsis compared with other sedatives. Dexmedetomidine might lead to an increased incidence of arrhythmias, but its safety profile did not show significant differences in the incidence of total adverse events. Future RCTs are needed to determine the sepsis patient population that would benefit most from dexmedetomidine and its optimal dosing regimen.
Keywords: Dexmedetomidine; Inflammatory response; Intensive critical care; Meta-analysis; Mortality; Sedatives; Sepsis; Survival.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, Angus DC, Reinhart K. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193(3):259–272. doi: 10.1164/rccm.201504-0781OC. - DOI - PubMed
-
- Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, McIntyre L, Ostermann M, Prescott HC, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181–1247. doi: 10.1007/s00134-021-06506-y. - DOI - PMC - PubMed
-
- The First ESA European Sepsis Report — European Sepsis Alliance. https://www.europeansepsisalliance.org/news/2021/9/9/the-first-esa-europ....
-
- Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200–211. doi: 10.1016/S0140-6736(19)32989-7. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources