Efficient infection of non-human primates with purified, cryopreserved Plasmodium knowlesi sporozoites
- PMID: 36030292
- PMCID: PMC9418655
- DOI: 10.1186/s12936-022-04261-z
Efficient infection of non-human primates with purified, cryopreserved Plasmodium knowlesi sporozoites
Abstract
Background: Plasmodium falciparum (Pf) sporozoite (SPZ) vaccines are the only candidate malaria vaccines that induce > 90% vaccine efficacy (VE) against controlled human malaria infection and the only malaria vaccines to have achieved reproducible VE against malaria in adults in Africa. The goal is to increase the impact and reduce the cost of PfSPZ vaccines by optimizing vaccine potency and manufacturing, which will benefit from identification of immunological responses contributing to protection in humans. Currently, there is no authentic animal challenge model for assessing P. falciparum malaria VE. Alternatively, Plasmodium knowlesi (Pk), which infects humans and non-human primates (NHPs) in nature, can be used to experimentally infect rhesus macaques (Macaca mulatta) to assess VE.
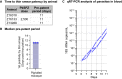
Methods: Sanaria has, therefore, produced purified, vialed, cryopreserved PkSPZ and conducted challenge studies in several naïve NHP cohorts. In the first cohort, groups of three rhesus macaques each received doses of 5 × 102, 2.5 × 103, 1.25 × 104 and 2.5 × 104 PkSPZ administered by direct venous inoculation. The infectivity of 1.5 × 103 PkSPZ cryopreserved with an altered method and of 1.5 × 103 PkSPZ cryopreserved for four years was tested in a second and third cohort of rhesus NHPs. The lastly, three pig-tailed macaques (Macaca nemestrina), a natural P. knowlesi host, were challenged with 2.5 × 103 PkSPZ cryopreserved six years earlier.
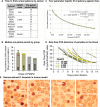
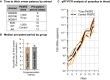
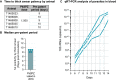
Results: In the first cohort, all 12 animals developed P. knowlesi parasitaemia by thick blood smear, and the time to positivity (prepatent period) followed a non-linear 4-parameter logistic sigmoidal model with a median of 11, 10, 8, and 7 days, respectively (r2 = 1). PkSPZ cryopreserved using a modified rapid-scalable method infected rhesus with a pre-patent period of 10 days, as did PkSPZ cryopreserved four years prior to infection, similar to the control group. Cryopreserved PkSPZ infected pig-tailed macaques with median time to positivity by thin smear, of 11 days.
Conclusion: This study establishes the capacity to consistently infect NHPs with purified, vialed, cryopreserved PkSPZ, providing a foundation for future studies to probe protective immunological mechanisms elicited by PfSPZ vaccines that cannot be established in humans.
© 2022. The Author(s).
Conflict of interest statement
S.C., E.R.J., U.R., N.K.C., and S.L.H. are employees of Sanaria Inc, and draw their salaries from the company.
Figures
References
-
- Shekalaghe S, Rutaihwa M, Billingsley PF, Chemba M, Daubenberger CA, James ER, et al. Controlled human malaria infection of tanzanians by intradermal injection of aseptic, purified, cryopreserved Plasmodium falciparum sporozoites. Am J Trop Med Hyg. 2014;91:471–80. doi: 10.4269/ajtmh.14-0119. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

