Pilot dose-ranging of rhIGF-1/rhIGFBP-3 in a preterm lamb model of evolving bronchopulmonary dysplasia
- PMID: 36030318
- PMCID: PMC9968819
- DOI: 10.1038/s41390-022-02272-9
Pilot dose-ranging of rhIGF-1/rhIGFBP-3 in a preterm lamb model of evolving bronchopulmonary dysplasia
Abstract
Background: Low levels of insulin-like growth factor-1 (IGF-1) protein in preterm human infants are associated with bronchopulmonary dysplasia (BPD). We used our preterm lamb model of BPD to determine (1) dosage of recombinant human (rh) IGF-1 bound to binding protein-3 (IGFBP-3) to reach infant physiologic plasma levels; and (2) whether repletion of plasma IGF-1 improves pulmonary and cardiovascular outcomes.
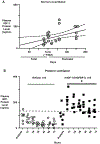
Methods: Group 1: normal, unventilated lambs from 128 days gestation through postnatal age 5 months defined normal plasma levels of IGF-1. Group 2: continuous infusion of rhIGF-1/rhIGFBP-3 (0.5, 1.5, or 4.5 mg/kg/day; n = 2) for 3 days in mechanically ventilated (MV) preterm lambs determined that 1.5 mg/kg/day dosage attained physiologic plasma IGF-1 concentration of ~125 ng/mL, which was infused in four more MV preterm lambs.
Results: Group 1: plasma IGF-1 protein increased from ~75 ng/mL at 128 days gestation to ~220 ng/L at 5 months. Group 2: pilot study of the optimal dosage (1.5 mg/kg/day rhIGF-1/rhIGFBP-3) in six MV preterm lambs significantly improved some pulmonary and cardiovascular outcomes (p < 0.1) compared to six MV preterm controls. RhIGF-1/rhIGFBP-3 was not toxic to the liver, kidneys, or lungs.
Conclusions: Three days of continuous iv infusion of rhIGF-1/rhIGFBP-3 at 1.5 mg/kg/day improved some pulmonary and cardiovascular outcomes without toxicity.
Impact: Preterm birth is associated with rapid decreases in serum or plasma IGF-1 protein level. This decline adversely impacts the growth and development of the lung and cardiovascular system. For this pilot study, continuous infusion of optimal dosage of rhIGF-1/rhIGFBP-3 (1.5 mg/kg/day) to maintain physiologic plasma IGF-1 level of ~125 ng/mL during mechanical ventilation for 3 days statistically improved some structural and biochemical outcomes related to the alveolar formation that would favor improved gas exchange compared to vehicle-control. We conclude that 3 days of continuous iv infusion of rhIGF-1/rhIGFBP-3 improved some physiological, morphological, and biochemical outcomes, without toxicity, in mechanically ventilated preterm lambs.
© 2022. The Author(s), under exclusive licence to the International Pediatric Research Foundation, Inc.
Figures
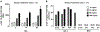



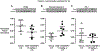
References
-
- Northway WH Jr., Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. New Eng J Med 1967;276:357–368. - PubMed
-
- Olmos A, et al. Associations between insulin-like growth factor I, vascular endothelial growth factor and its soluble receptor 1 in umbilical serum and endothelial cells obtained from normotensive and preeclamptic pregnancies. Growth Factors 2013;31:123–129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

