Ondansetron to reduce neonatal opioid withdrawal severity a randomized clinical trial
- PMID: 36030327
- PMCID: PMC9968817
- DOI: 10.1038/s41372-022-01487-2
Ondansetron to reduce neonatal opioid withdrawal severity a randomized clinical trial
Abstract
Objective: To determine if treatment with a 5-HT3 antagonist (ondansetron) reduces need for opioid therapy in infants at risk for neonatal opioid withdrawal syndrome (NOWS).
Study design: A multicenter, randomized, placebo controlled, double blind clinical trial of ninety (90) infants. The intervention arms were intravenous ondansetron or placebo during labor followed by a daily dose of ondansetron or placebo in infants for five days.
Results: Twenty-two (49%) ondansetron-treated and 26 (63%) placebo-treated infants required pharmacologic treatment (p > 0.05). The Finnegan score was lower in the ondansetron-treated group (4.6 vs. 5.6, p = 0.02). A non-significant trend was noted for the duration of hospitalization. There was no difference in need for phenobarbital or clonidine therapy, or total dose of morphine in the first 15 days of NOWS treatment.
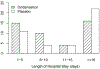
Conclusions: Ondansetron treatment reduced the severity of NOWS symptoms; and there was an indication that it could reduce the length of stay.
Clinical trial registration: Clinicaltrials.gov NCT01965704.
© 2022. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Conflict of interest disclosure
The authors have no conflicts of interest relevant to this article to disclose.
Figures



References
-
- Peltz G, Sudhof TC. The Neurobiology of Opioid Addiction and the Potential for Prevention Strategies. JAMA 2018. May 22;319(20):2071–2072. - PubMed
-
- Erlendson MJ, D’Arcy N, Encisco EM, Yu JJ, Rincon-Cruz L, Peltz G, et al. Palonosetron and hydroxyzine pre-treatment reduces the objective signs of experimentally-induced acute opioid withdrawal in humans: a double-blinded, randomized, placebo-controlled crossover study. Am J Drug Alcohol Abuse 2017. Jan;43(1):78–86. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

