Device-related complications in subcutaneous versus transvenous ICD: a secondary analysis of the PRAETORIAN trial
- PMID: 36030464
- PMCID: PMC9748587
- DOI: 10.1093/eurheartj/ehac496
Device-related complications in subcutaneous versus transvenous ICD: a secondary analysis of the PRAETORIAN trial
Abstract
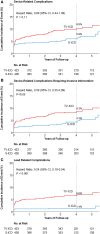
Background: The subcutaneous implantable cardioverter-defibrillator (S-ICD) is developed to overcome lead-related complications and systemic infections, inherent to transvenous ICD (TV-ICD) therapy. The PRAETORIAN trial demonstrated that the S-ICD is non-inferior to the TV-ICD with regard to the combined primary endpoint of inappropriate shocks and complications. This prespecified secondary analysis evaluates all complications in the PRAETORIAN trial.
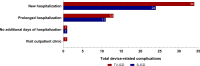
Methods and results: The PRAETORIAN trial is an international, multicentre, randomized trial in which 849 patients with an indication for ICD therapy were randomized to receive an S- ICD (N = 426) or TV-ICD (N = 423) and followed for a median of 49 months. Endpoints were device-related complications, lead-related complications, systemic infections, and the need for invasive interventions. Thirty-six device-related complications occurred in 31 patients in the S-ICD group of which bleedings were the most frequent. In the TV-ICD group, 49 complications occurred in 44 patients of which lead dysfunction was most frequent (HR: 0.69; P = 0.11). In both groups, half of all complications were within 30 days after implantation. Lead-related complications and systemic infections occurred significantly less in the S-ICD group compared with the TV-ICD group (P < 0.001, P = 0.03, respectively). Significantly more complications required invasive interventions in the TV-ICD group compared with the S-ICD group (8.3% vs. 4.3%, HR: 0.59; P = 0.047).
Conclusion: This secondary analysis shows that lead-related complications and systemic infections are more prevalent in the TV-ICD group compared with the S-ICD group. In addition, complications in the TV-ICD group were more severe as they required significantly more invasive interventions. This data contributes to shared decision-making in clinical practice.
Keywords: Complications; Infections; Invasive interventions; Lead-related complications; Subcutaneous ICD; Transvenous ICD.
© The Author(s) 2022. Published by Oxford University Press on behalf of European Society of Cardiology.
Conflict of interest statement
Conflict of interest: R.E.K reports consultancy fees and research grants from Abbott, Boston Scientific, Medtronic, and Cairdac and has stock options from AtaCor Medical Inc. S.M. reports consultancy fees from Boston Scientific. K.V. reports consultancy fees from Medtronic and Abbott. M.C.B. is a consultant and receives honoraria, as well as research grants from Boston Scientific and has equity in and is chief medical officer for AtaCor Medical, Inc. D.J.W. has consultancy arrangements with Boston Scientific and Medtronic and a research grant from Boston Scientific. P.N. reports modest speaker honoraria from Biotronik, Boston Scientific, and Medtronic. M.A.M. reports consultancy fees from Boston Scientific. Z.I.W. is an advisor for Boston Scientific and on the advisory board for Medtronic and Abbot and reports speaker fees from Medtronic. The other authors report no conflicts.
Figures


Comment in
-
Subcutaneous implantable cardioverter defibrillator for the prevention of sudden cardiac death: ready for prime-time?Eur Heart J. 2022 Dec 14;43(47):4884-4886. doi: 10.1093/eurheartj/ehac652. Eur Heart J. 2022. PMID: 36380686 No abstract available.
References
-
- Tarakji KG, Wazni OM, Harb S, et al. Risk factors for 1-year mortality among patients with cardiac implantable electronic device infection undergoing transvenous lead extraction: the impact of the infection type and the presence of vegetation on survival. Europace 2014;16:1490–1495. - PubMed
-
- Sohail MR, Uslan DZ, Khan AH, et al. Management and outcome of permanent pacemaker and implantable cardioverter-defibrillator infections. J Am Coll Cardiol 2007;49:1851–1859. - PubMed
-
- Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877–883. - PubMed
-
- Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225–237. - PubMed

