IL-34 deficiency impairs FOXP3+ Treg function in a model of autoimmune colitis and decreases immune tolerance homeostasis
- PMID: 36030499
- PMCID: PMC9420423
- DOI: 10.1002/ctm2.988
IL-34 deficiency impairs FOXP3+ Treg function in a model of autoimmune colitis and decreases immune tolerance homeostasis
Abstract
Background: Immune homeostasis requires fully functional Tregs with a stable phenotype to control autoimmunity. Although IL-34 is a cytokine first described as mainly involved in monocyte cell survival and differentiation, we recently described its expression by CD8+ Tregs in a rat model of transplantation tolerance and by activated FOXP3+ CD4+ and CD8+ Tregs in human healthy individuals. However, its role in autoimmunity and potential in human diseases remains to be determined.
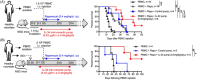
Methods: We generated Il34-/- rats and using both Il34-/- rats and mice, we investigated their phenotype under inflammatory conditions. Using Il34-/- rats, we further analyzed the impact of the absence of expression of IL-34 for CD4+ Tregs suppressive function. We investigated the potential of IL-34 in human disease to prevent xenogeneic GVHD and human skin allograft rejection in immune humanized immunodeficient NSG mice. Finally, taking advantage of a biocollection, we investigated the correlation between presence of IL-34 in the serum and kidney transplant rejection.
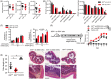
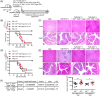
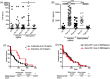
Results: Here we report that the absence of expression of IL-34 in Il34-/- rats and mice leads to an unstable immune phenotype, with production of multiple auto-antibodies, exacerbated under inflammatory conditions with increased susceptibility to DSS- and TNBS-colitis in Il34-/- animals. Moreover, we revealed the striking inability of Il34-/- CD4+ Tregs to protect Il2rg-/- rats from a wasting disease induced by transfer of pathogenic cells, in contrast to Il34+/+ CD4+ Tregs. We also showed that IL-34 treatment delayed EAE in mice as well as GVHD and human skin allograft rejection in immune humanized immunodeficient NSG mice. Finally, we show that presence of IL-34 in the serum is associated with a longer rejection-free period in kidney transplanted patients.
Conclusion: Altogether, our data emphasize on the crucial necessity of IL-34 for immune homeostasis and for CD4+ Tregs suppressive function. Our data also shows the therapeutic potential of IL-34 in human transplantation and auto-immunity.
Highlights: -Absence of expression of IL-34 in Il34-/- rats and mice leads to an unstable immune phenotype, with a production of multiple auto-antibodies and exacerbated immune pathology under inflammatory conditions. -Il34-/- CD4+ Tregs are unable to protect Il2rg-/- rats from colitis induced by transfer of pathogenic cells. -IL-34 treatment delayed EAE in mice, as well as acute GVHD and human skin allograft rejection in immune-humanized immunodeficient NSG mice.
Keywords: CRISPR/Cas9; Foxp3; IL-34; Treg; autoimmunity; immunotherapy; knockout; rat; tolerance.
© 2022 The Authors. Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
C.G., I.A. and S.B. have patents registered on IL‐34.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
