Neuronavigation maximizes accuracy and precision in TMS positioning: Evidence from 11,230 distance, angle, and electric field modeling measurements
- PMID: 36031059
- PMCID: PMC10026380
- DOI: 10.1016/j.brs.2022.08.013
Neuronavigation maximizes accuracy and precision in TMS positioning: Evidence from 11,230 distance, angle, and electric field modeling measurements
Abstract
Background: Researchers and clinicians have traditionally relied on elastic caps with markings to reposition the transcranial magnetic stimulation (TMS) coil between trains and sessions. Newer neuronavigation technology co-registers the patient's head and structural magnetic resonance imaging (MRI) scan, providing the researcher with real-time feedback about how to adjust the coil to be on-target. However, there has been no head to head comparison of accuracy and precision across treatment sessions.
Objective: /Hypothesis: In this two-part study, we compared elastic cap and neuronavigation targeting methodologies on distance, angle, and electric field (E-field) magnitude values.
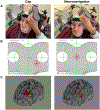
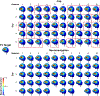
Methods: In 42 participants receiving up to 50 total accelerated rTMS sessions in 5 days, we compared cap and neuronavigation targeting approaches in 3408 distance and 6816 angle measurements. In Experiment 1, TMS administrators saved an on-target neuronavigation location at Beam F3, which served as the landmark for all other measurements. Next, the operators placed the TMS coil based on cap markings or neuronavigation software to measure the distance and angle differences from the on-target sample. In Experiment 2, we saved each XYZ coordinate of the TMS coil from cap and neuronavigation targeting in 12 participants to compare the E-field magnitude differences at the cortical prefrontal target in 1106 cap and neuronavigation models.
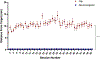
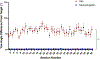
Results: Cap targeting was significantly off-target for distance, placing the coil an average of 10.66 mm off-target (Standard error of the mean; SEM = 0.19 mm) compared to 0.3 mm (SEM = 0.03 mm) for neuronavigation (p < 0.0001). Cap targeting also significantly deviated for angles off-target, averaging 7.79 roll/pitch degrees (SEM = 1.07°) off-target and 5.99 yaw degrees (SEM = 0.12°) off-target; in comparison, neuronavigation targeting positioned the coil 0.34 roll/pitch degrees (SEM = 0.01°) and 0.22 yaw (SEM = 0.004°) off-target (both p < 0.0001). Further analyses revealed that there were significant inter-operator differences on distance and angle positioning for F3 (all p < 0.05), but not neuronavigation. Lastly, cap targeting resulted in significantly lower E-fields at the intended prefrontal cortical target, with equivalent E-fields as 110.7% motor threshold (MT; range = 58.3-127.4%) stimulation vs. 119.9% MT (range = 115-123.3%) from neuronavigated targeting with 120% MT stimulation applied (p < 0.001).
Conclusions: Cap-based targeting is an inherent source of target variability compared to neuronavigation. Additionally, cap-based coil placement is more prone to differences across operators. Off-target coil placement secondary to cap-based measurements results in significantly lower amounts of stimulation reaching the cortical target, with some individuals receiving only 48.6% of the intended on-target E-field. Neuronavigation technology enables more precise and accurate TMS positioning, resulting in the intended stimulation intensities at the targeted cortical level.
Keywords: Dorsolateral prefrontal cortex (DLPFC); Elastic cap targeting; Electric field (E-field) modeling; Neuronavigated rTMS; Neuronavigation; Transcranial magnetic stimulation.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest We confirm that there are no known conflicts of interest associated with this publication and there was no financial support for this work that could have influenced its outcome.
Figures




References
-
- O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatr 2007;62(11):1208–16. - PubMed
-
- George MS, Lisanby SH, Avery D, McDonald WM, Durkalski V, Pavlicova M, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatr 2010;67(5):507–16. - PubMed
-
- Carmi L, Tendler A, Bystritsky A, Hollander E, Blumberger DM, Daskalakis J, et al. Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: a prospective multicenter randomized double-blind placebo-controlled trial. Am J Psychiatr 2019;176(11):931–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

