Early screening for gestational diabetes mellitus: a meta-analysis of randomized controlled trials
- PMID: 36031150
- PMCID: PMC11491265
- DOI: 10.1016/j.ajogmf.2022.100737
Early screening for gestational diabetes mellitus: a meta-analysis of randomized controlled trials
Abstract
Objective: Current evidence is conflicting on whether early screening and treatment for gestational diabetes mellitus improve pregnancy outcomes. Thus, this systematic review and meta-analysis of randomized controlled trials aimed to assess the rate of adverse pregnancy outcomes among participants with early screening and treatment for gestational diabetes mellitus vs those with routine care.
Data sources: A systematic review of the literature was conducted using MEDLINE, Scopus, ClinicalTrials.gov, EMBASE, ScienceDirect, the Cochrane Library at the Central Register of Controlled Trials, and SciELO from inception to November 2021.
Study eligibility criteria: Studies were eligible for inclusion if they described randomized controlled trials comparing early screening with routine care for gestational diabetes mellitus to assess the effects of early screening and treatment on pregnancy outcomes.
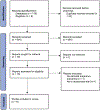
Methods: All randomized controlled trials comparing early vs standard screening of gestational diabetes mellitus assessing the effect of early screening (defined as a screening at <20 weeks of gestation) vs routine screening (defined as a screening at ≥20 weeks of gestation) on pregnancy outcomes were included. The primary outcome was defined as large for gestational age, as defined by the trial. The secondary maternal and neonatal outcomes were also evaluated. Subgroup analyses were performed on the basis of screening strategy and methods.
Results: After exclusion, 8 randomized clinical trials (1920 participants) of early screening and treatment vs standard care were included. There were a total of 746 participants with early gestational diabetes mellitus. The risk of large for gestational age at birth did not differ between early screening and treatment for gestational diabetes mellitus and routine care among all included trials (8.1 vs 9.0%; relative risk, 0.94; 95% confidence interval, 0.73-1.22). Trials with a protocol of universal screening of participants at their first prenatal visit (>80% screened with HbA1c) and receiving early treatment if the screening test returned positive had a lower risk of large for gestational age (2.3 vs 9.1%; relative risk, 0.29; 95% confidence interval, 0.09-0.90) than those who had routine screening and care.
Conclusion: Overall, early screening and treatment of gestational diabetes mellitus did not reduce the risk of large for gestational age at birth. However, trials that screened all participants at their first visit and treated early, most for an HbA1c of 5.7% to 6.4%, had a reduced risk of large for gestational age at birth compared with routine care, suggesting a possible benefit of screening all pregnant patients. However, future well-designed trials are needed to confirm these findings.
Keywords: diabetes mellitus; early screening; gestational diabetes mellitus; large for gestational age; macrosomia.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors report no conflict of interest.
Figures

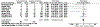
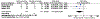
References
-
- ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol 2018;131: e49–64. - PubMed
-
- Yogev Y, Xenakis EM, Langer O. The association between preeclampsia and the severity of gestational diabetes: the impact of glycemic control. Am J Obstet Gynecol 2004;191:1655–60. - PubMed
-
- Ehrenberg HM, Durnwald CP, Catalano P, Mercer BM. The influence of obesity and diabetes on the risk of cesarean delivery. Am J Obstet Gynecol 2004;191:969–74. - PubMed
-
- HAPO Study Cooperative Research Group-Metzger BE, Lowe LP, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002. - PubMed

