New Deferric Amine Compounds Efficiently Chelate Excess Iron to Treat Iron Overload Disorders and to Prevent Ferroptosis
- PMID: 36031399
- PMCID: PMC9561787
- DOI: 10.1002/advs.202202679
New Deferric Amine Compounds Efficiently Chelate Excess Iron to Treat Iron Overload Disorders and to Prevent Ferroptosis
Abstract
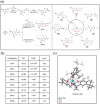
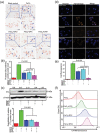
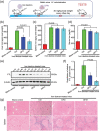
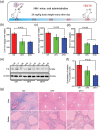
Excess iron accumulation occurs in organs of patients with certain genetic disorders or after repeated transfusions. No physiological mechanism is available to excrete excess iron and iron overload to promote lipid peroxidation to induce ferroptosis, thus iron chelation becomes critical for preventing ion toxicity in these patients. To date, several iron chelators have been approved for iron chelation therapy, such as deferiprone and deferoxamine, but the current iron chelators suffer from significant limitations. In this context, new agents are continuously sought. Here, a library of new deferric amine compounds (DFAs) with adjustable skeleton and flexibility is synthesized by adopting the beneficial properties of conventional chelators. After careful evaluations, compound DFA1 is found to have greater efficacy in binding iron through two molecular oxygens in the phenolic hydroxyl group and the nitrogen atom in the amine with a 2:1 stoichiometry. This compound remarkably ameliorates iron overload in diverse murine models through both oral and intravenous administration, including hemochromatosis, high iron diet-induced, and iron dextran-stimulated iron accumulation. Strikingly, this compound is found to suppress iron-induced ferroptosis by modulating the intracellular signaling that drives lipid peroxidation. This study opens a new approach for the development of iron chelators to treat iron overload.
Keywords: deferric amine compound; ferroptosis; iron chelator; iron overload; liver injury.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- a) Adams P. C., Reboussin D. M., Barton J. C., McLaren C. E., Eckfeldt J. H., McLaren G. D., Dawkins F. W., Acton R. T., Harris E. L., Gordeuk V. R., Leiendecker‐Foster C., Speechley M., Snively B. M., Holup J. L., Thomson E., Sholinsky P., Acton R. T., Barton J. C., Dixon D., Rivers C. A., Tucker D., Ware J. C., McLaren C. E., McLaren G. D., Anton‐Culver H., Baca J. A., Bent T. C., Brunner L. C., Dao M. M., Jorgensen K. S., et al., N. Engl. J. Med. 2005, 352, 1769; - PubMed
- b) Allen K. J., Gurrin L. C., Constantine C. C., Osborne N. J., Delatycki M. B., Nicoll A. J., McLaren C. E., Bahlo M., Nisselle A. E., Vulpe C. D., Anderson G. J., Southey M. C., Giles G. G., English D. R., Hopper J. L., Olynyk J. K., Powell L. W., Gertig D. M., N. Engl. J. Med. 2008, 358, 221; - PubMed
- c) Pietrangelo A., N. Engl. J. Med. 2004, 350, 2383. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 21920102007/National Natural Science Foundation of China
- 22150006/National Natural Science Foundation of China
- 22076210/National Natural Science Foundation of China
- 2021YFE0101500/Ministry of Science and Technology of the People's Republic of China
- 121311KYSB20190010/International Collaboration Key Grant from the Chinese Academy of Sciences
