Extracellular Hydraulic Resistance Enhances Cell Migration
- PMID: 36031406
- PMCID: PMC9561764
- DOI: 10.1002/advs.202200927
Extracellular Hydraulic Resistance Enhances Cell Migration
Abstract
Cells migrating in vivo encounter microenvironments with varying physical properties. One such physical variable is the fluid viscosity surrounding the cell. Increased viscosity is expected to increase the hydraulic resistance experienced by the cell and decrease cell speed. The authors demonstrate that contrary to this expected result, cells migrate faster in high viscosity media on 2-dimensional substrates. Both actin dynamics and water dynamics driven by ion channel activity are examined. Results show that cells increase in area in high viscosity and actomyosin dynamics remain similar. Inhibiting ion channel fluxes in high viscosity media results in a large reduction in cell speed, suggesting that water flux contributes to the observed speed increase. Moreover, inhibiting actin-dependent vesicular trafficking that transports ion channels to the cell boundary changes ion channel spatial positioning and reduces cell speed in high viscosity media. Cells also display altered Ca2+ activity in high viscosity media, and when cytoplasmic Ca2+ is sequestered, cell speed reduction and altered ion channel positioning are observed. Taken together, it is found that the cytoplasmic actin-phase and water-phase are coupled to drive cell migration in high viscosity media, in agreement with physical modeling that also predicts the observed cell speedup in high viscosity environments.
Keywords: cancer; cell migration; osmotic engine model; tumor microenvironment; viscosity.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


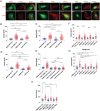

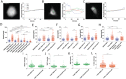
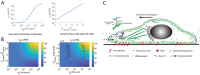
References
-
- Trepat X., Chen Z., Jacobson K., in Comprehensive Physiology, Vol. 176, John Wiley & Sons, Inc., 2012, pp. 139–148.
-
- Vicente‐Manzanares M., Webb D. J., Horwitz A. R., J. Cell Sci. 2005, 118, 4917. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
