Emerging strategies in targeting tumor-resident myeloid cells for cancer immunotherapy
- PMID: 36031601
- PMCID: PMC9420297
- DOI: 10.1186/s13045-022-01335-y
Emerging strategies in targeting tumor-resident myeloid cells for cancer immunotherapy
Abstract
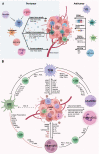
Immune checkpoint inhibitors targeting programmed cell death protein 1, programmed death-ligand 1, and cytotoxic T-lymphocyte-associated protein 4 provide deep and durable treatment responses which have revolutionized oncology. However, despite over 40% of cancer patients being eligible to receive immunotherapy, only 12% of patients gain benefit. A key to understanding what differentiates treatment response from non-response is better defining the role of the innate immune system in anti-tumor immunity and immune tolerance. Teleologically, myeloid cells, including macrophages, dendritic cells, monocytes, and neutrophils, initiate a response to invading pathogens and tissue repair after pathogen clearance is successfully accomplished. However, in the tumor microenvironment (TME), these innate cells are hijacked by the tumor cells and are imprinted to furthering tumor propagation and dissemination. Major advancements have been made in the field, especially related to the heterogeneity of myeloid cells and their function in the TME at the single cell level, a topic that has been highlighted by several recent international meetings including the 2021 China Cancer Immunotherapy workshop in Beijing. Here, we provide an up-to-date summary of the mechanisms by which major myeloid cells in the TME facilitate immunosuppression, enable tumor growth, foster tumor plasticity, and confer therapeutic resistance. We discuss ongoing strategies targeting the myeloid compartment in the preclinical and clinical settings which include: (1) altering myeloid cell composition within the TME; (2) functional blockade of immune-suppressive myeloid cells; (3) reprogramming myeloid cells to acquire pro-inflammatory properties; (4) modulating myeloid cells via cytokines; (5) myeloid cell therapies; and (6) emerging targets such as Siglec-15, TREM2, MARCO, LILRB2, and CLEVER-1. There is a significant promise that myeloid cell-based immunotherapy will help advance immuno-oncology in years to come.
Keywords: CAR-M; Dendritic cells; Myeloid; Myeloid-derived suppressor cells (MDSCs); Polarization; Proliferation; Recruitment; Reprogramming; Tumor microenvironment; Tumor-associated macrophages (TAMs).
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests related to the words discussed.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

