Computation of contrast-enhanced perfusion using only two CT scan phases: a proof-of-concept study on abdominal organs
- PMID: 36031643
- PMCID: PMC9420683
- DOI: 10.1186/s41747-022-00292-y
Computation of contrast-enhanced perfusion using only two CT scan phases: a proof-of-concept study on abdominal organs
Abstract
Background: Computed tomography perfusion imaging (CTPI) by repeated scanning has clinical relevance but implies relatively high radiation exposure. We present a method to measure perfusion from two CT scan phases only, considering tissue enhancement, feeding vessel (aortic) peak enhancement, and bolus shape.
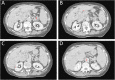
Methods: CTPI scans (each with 40 frames acquired every 1.5 s) of 11 patients with advanced hepatocellular carcinoma (HCC) enrolled between 2012 and 2016 were retrospectively analysed (aged 69 ± 9 years, 8/11 males). Perfusion was defined as the maximal slope of the time-enhancement curve divided by the peak enhancement of the feeding vessel (aorta). Perfusion was computed two times, first using the maximum slope derived from all data points and then using the peak tissue enhancement and the bolus shape obtained from the aortic curve.
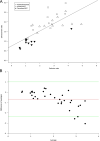
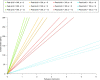
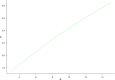
Results: Perfusion values from the two methods were linearly related (r2 = 0.92, p < 0.001; Bland-Altman analysis bias -0.12). The mathematical model showed that the perfusion ratio of two ROIs with the same feeding vessel (aorta) corresponds to their peak enhancement ratio (r2 = 0.55, p < 0.001; Bland-Altman analysis bias -0.68). The relationship between perfusion and tissue enhancement is predicted to be linear in the clinical range of interest, being only function of perfusion, peak feeding vessel enhancement, and bolus shape.
Conclusions: This proof-of-concept study showed that perfusion values of HCC, kidney, and pancreas could be computed using enhancement measured only with two CT scan phases, if aortic peak enhancement and bolus shape are known.
Keywords: Carcinoma (hepatocellular); Contrast media; Perfusion imaging; Tomography (x-ray computed).
© 2022. The Author(s) under exclusive licence to European Society of Radiology.
Conflict of interest statement
Massimo Cressoni, Paolo Cadringher, Paolo Vitali, Gianpaolo Basso, and Davide Ippolito all declare that they have no conflict of interest related to the present work.
Simone Schiaffino received travel support from Bracco Imaging and is a member of the speakers’ bureau for General Electric Healthcare.
Francesco Sardanelli received research grants from—and is a member of the speakers’ bureau of—General Electric Healthcare, Bayer, and Bracco; he is also member of the Bracco Advisory Group.
Andrea Cozzi and Simone Schiaffino are members of the Editorial Board of
Figures


References
-
- Ippolito D, Querques G, Okolicsanyi S, et al. Dynamic contrast enhanced perfusion CT imaging: a diagnostic biomarker tool for survival prediction of tumour response to antiangiogenetic treatment in patients with advanced HCC lesions. Eur J Radiol. 2018;106:62–68. doi: 10.1016/j.ejrad.2018.07.012. - DOI - PubMed
