Discovery of an L-like Configuration for 3'-Fluoro-5'-norcarbonucleoside Phosphonates as Potent Anti-HIV Agents
- PMID: 36032023
- PMCID: PMC9825896
- DOI: 10.1002/cmdc.202200377
Discovery of an L-like Configuration for 3'-Fluoro-5'-norcarbonucleoside Phosphonates as Potent Anti-HIV Agents
Abstract
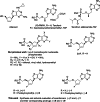
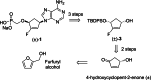
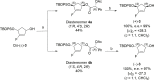
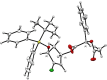
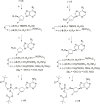
Recently, we reported the racemic synthesis of 3'-fluoro-5'-norcarbocyclic nucleoside phosphonates bearing adenine as the heterocyclic base. For this study, to evaluate the antiviral activity of each enantiomer, we synthesized both enantiomers, as well as their corresponding bis(POM) prodrugs. Anti-HIV-1 evaluation against the LAI strain and clinically NRTI-resistant HIV-1 strains are presented. The activities against these different strains show that the activities of bis(POM) prodrug (-)-9 are equivalent or even superior to those of (R)-PMPA.
Keywords: HIV; L-enantiomer; antiviral; nucleoside; phosphonate.
© 2022 The Authors. ChemMedChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Beyrer C., The Lancet 2021, 397, 2142–2143. - PubMed
-
- None
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

