M2 Macrophages promote IL-33 expression, ILC2 expansion and mucous metaplasia in response to early life rhinovirus infections
- PMID: 36032072
- PMCID: PMC9412168
- DOI: 10.3389/fimmu.2022.952509
M2 Macrophages promote IL-33 expression, ILC2 expansion and mucous metaplasia in response to early life rhinovirus infections
Abstract
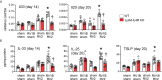
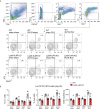
Wheezing-associated rhinovirus (RV) infections are associated with asthma development. We have shown that infection of immature mice with RV induces type 2 cytokine production and mucous metaplasia which is dependent on IL-33 and type 2 innate lymphoid cells (ILC2s) and intensified by a second heterologous RV infection. We hypothesize that M2a macrophages are required for the exaggerated inflammation and mucous metaplasia in response to heterologous RV infection. Wild-type C57Bl/6J mice and LysMCre IL4Rα KO mice lacking M2a macrophages were treated as follows: (1) sham infection on day 6 of life plus sham on day 13 of life, (2) RV-A1B on day 6 plus sham on day 13, (3) sham on day 6 and RV-A2 on day 13, or (4) RV-A1B on day 6 and RV-A2 on day 13. Lungs were harvested one or seven days after the second infection. Wild-type mice infected with RV-A1B at day 6 showed an increased number of Arg1- and Retnla-expressing lung macrophages, indicative of M2a polarization. Compared to wild-type mice infected with RV on day 6 and 13 of life, the lungs of LysMCre IL4Rα KO mice undergoing heterologous RV infection showed decreased protein abundance of the epithelial-derived innate cytokines IL-33, IL-25 and TSLP, decreased ILC2s, decreased mRNA expression of IL-13 and IL-5, and decreased PAS staining. Finally, mRNA analysis and immunofluorescence microscopy of double-infected LysMCre IL4Rα KO mice showed reduced airway epithelial cell IL-33 expression, and treatment with IL-33 restored the exaggerated muco-inflammatory phenotype.
Conclusion: Early-life RV infection alters the macrophage response to subsequent heterologous infection, permitting enhanced IL-33 expression, ILC2 expansion and intensified airway inflammation and mucous metaplasia.
Keywords: IL-33; ILC2; M2 macrophage; Rhinovirus; neonate.
Copyright © 2022 Han, Breckenridge, Kuo, Singh, Goldsmith, Li, Kreger, Bentley and Hershenson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

