Development and characterization of a CRISPR/Cas9-mediated RAG1 knockout chicken model lacking mature B and T cells
- PMID: 36032098
- PMCID: PMC9403712
- DOI: 10.3389/fimmu.2022.892476
Development and characterization of a CRISPR/Cas9-mediated RAG1 knockout chicken model lacking mature B and T cells
Abstract
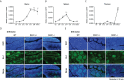
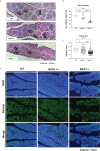
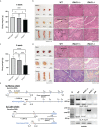
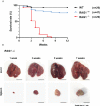
Although birds have been used historically as a model animal for immunological research, resulting in remarkable achievements, immune cell development in birds themselves has yet to be fully elucidated. In this study, we firstly generated an immunodeficient chicken model using a CRISPR/Cas9-mediated recombination activating gene 1 (RAG1) knockout, to investigate avian-specific immune cell development. Unlike previously reported immunoglobulin (Ig) heavy chain knockout chickens, the proportion and development of B cells in both RAG1 +/- and RAG1 -/- embryos were significantly impaired during B cell proliferation (embryonic day 16 to 18). Our findings indicate that, this is likely due to disordered B cell receptor (BCR)-mediated signaling and interaction of CXC motif chemokine receptor (CXCR4) with CXCL12, resulting from disrupted Ig V(D)J recombination at the embryonic stage. Histological analysis after hatching showed that, unlike wild-type (WT) and RAG1 +/- chickens, lymphatic organs in 3-week old RAG1 -/- chickens were severely damaged. Furthermore, relative to WT chickens, RAG1+/- and RAG1-/- birds had reduced serum Igs, fewer mature CD4+ and CD8+ T lymphocytes. Furthermore, BCR-mediated B cell activation in RAG1 +/- chickens was insufficient, leading to decreased expression of the activation-induced deaminase (AID) gene, which is important for Ig gene conversion. Overall, this immunodeficient chicken model underlines the pivotal role of RAG1 in immature B cell development, Ig gene conversion during embryonic stages, and demonstrates the dose-dependent regulatory role of RAG1 during immune cell development. This model will provide ongoing insights for understanding chicken immune system development and applied in the fields of immunology and biomedical science.
Keywords: B cell; B cell receptor; CRISPR/Cas9; RAG1 knockout; T cell; avian immunology; embryonic stage; immunodeficient chicken.
Copyright © 2022 Lee, Choi, Park, Woo, Kim and Han.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Characterization of an N-Terminal Non-Core Domain of RAG1 Gene Disrupted Syrian Hamster Model Generated by CRISPR Cas9.Viruses. 2018 May 6;10(5):243. doi: 10.3390/v10050243. Viruses. 2018. PMID: 29734775 Free PMC article.
-
Characterization of rag1 mutant zebrafish leukocytes.BMC Immunol. 2009 Feb 3;10:8. doi: 10.1186/1471-2172-10-8. BMC Immunol. 2009. PMID: 19192305 Free PMC article.
-
Efficient Production of Biallelic RAG1 Knockout Mouse Embryonic Stem Cell Using CRISPR/Cas9.Iran J Biotechnol. 2019 Jan 11;17(1):e2205. doi: 10.21859/ijb.2205. eCollection 2019 Jan. Iran J Biotechnol. 2019. PMID: 31457047 Free PMC article.
-
CRISPR/Cas9 gene editing in a chicken model: current approaches and applications.J Appl Genet. 2020 May;61(2):221-229. doi: 10.1007/s13353-020-00537-9. J Appl Genet. 2020. PMID: 31925767 Free PMC article. Review.
-
RAG Chromatin Scanning During V(D)J Recombination and Chromatin Loop Extrusion are Related Processes.Adv Immunol. 2018;139:93-135. doi: 10.1016/bs.ai.2018.07.001. Epub 2018 Aug 27. Adv Immunol. 2018. PMID: 30249335 Review.
Cited by
-
Characterization and ontogeny of a novel lymphoid follicle inducer cell during development of the bursa of Fabricius.Front Immunol. 2024 Oct 21;15:1449117. doi: 10.3389/fimmu.2024.1449117. eCollection 2024. Front Immunol. 2024. PMID: 39497831 Free PMC article.
-
Conserved functional features of natural killer cell subsets in chicken, human, and murine immune systems.iScience. 2025 Jul 18;28(8):113144. doi: 10.1016/j.isci.2025.113144. eCollection 2025 Aug 15. iScience. 2025. PMID: 40792038 Free PMC article.
-
Advancement of animal and poultry nutrition: Harnessing the power of CRISPR-Cas genome editing technology.J Adv Vet Anim Res. 2024 Jun 21;11(2):483-493. doi: 10.5455/javar.2024.k798. eCollection 2024 Jun. J Adv Vet Anim Res. 2024. PMID: 39101073 Free PMC article.
-
Application of CRISPR/Cas gene editing for infectious disease control in poultry.Open Life Sci. 2025 May 20;20(1):20251095. doi: 10.1515/biol-2025-1095. eCollection 2025. Open Life Sci. 2025. PMID: 40417002 Free PMC article. Review.
-
Strategies for the Generation of Gene Modified Avian Models: Advancement in Avian Germline Transmission, Genome Editing, and Applications.Genes (Basel). 2023 Apr 12;14(4):899. doi: 10.3390/genes14040899. Genes (Basel). 2023. PMID: 37107658 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

