Development of RAG2 -/- IL2Rγ -/Y immune deficient FAH-knockout miniature pig
- PMID: 36032112
- PMCID: PMC9400017
- DOI: 10.3389/fimmu.2022.950194
Development of RAG2 -/- IL2Rγ -/Y immune deficient FAH-knockout miniature pig
Abstract
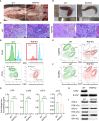
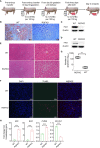
Human hepatocyte transplantation for liver disease treatment have been hampered by the lack of quality human hepatocytes. Pigs with their large body size, longevity and physiological similarities with human are appropriate animal models for the in vivo expansion of human hepatocytes. Here we report on the generation of RAG2-/-IL2Rγ-/YFAH-/- (RGFKO) pigs via CRISPR/Cas9 system and somatic cell nuclear transfer. We showed that thymic and splenic development in RGFKO pigs was impaired. V(D)J recombination processes were also inactivated. Consequently, RGFKO pigs had significantly reduced numbers of porcine T, B and NK cells. Moreover, due to the loss of FAH, porcine hepatocytes continuously undergo apoptosis and consequently suffer hepatic damage. Thus, RGFKO pigs are both immune deficient and constantly suffer liver injury in the absence of NTBC supplementation. These results suggest that RGFKO pigs have the potential to be engrafted with human hepatocytes without immune rejection, thereby allowing for large scale expansion of human hepatocytes.
Keywords: FAH; IL2Rγ; RAG2; immunodeficient; liver damage; pig.
Copyright © 2022 Zhao, Ye, Guo, Wang, Jiao, Xu, Yang, Chen, Jamal, Bai, Wei, Cai, Nguyen, Qing, Cheng, Jia, Li, Zhao, Chen and Wei.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Limited Expansion of Human Hepatocytes in FAH/RAG2-Deficient Swine.Tissue Eng Part A. 2022 Feb;28(3-4):150-160. doi: 10.1089/ten.TEA.2021.0057. Epub 2021 Oct 7. Tissue Eng Part A. 2022. PMID: 34309416 Free PMC article.
-
Efficient Generation of an Fah/Rag2 Dual-Gene Knockout Porcine Cell Line Using CRISPR/Cas9 and Adenovirus.DNA Cell Biol. 2019 Apr;38(4):314-321. doi: 10.1089/dna.2018.4493. Epub 2019 Feb 14. DNA Cell Biol. 2019. PMID: 30762444
-
Generation and characterization of RAG2 knockout pigs as animal model for severe combined immunodeficiency.Vet Immunol Immunopathol. 2016 Oct 1;178:37-49. doi: 10.1016/j.vetimm.2016.06.011. Epub 2016 Jun 30. Vet Immunol Immunopathol. 2016. PMID: 27496741
-
Fah Knockout Animals as Models for Therapeutic Liver Repopulation.Adv Exp Med Biol. 2017;959:215-230. doi: 10.1007/978-3-319-55780-9_20. Adv Exp Med Biol. 2017. PMID: 28755199 Review.
-
Small animal models of hepatocyte transplantation.Methods Mol Biol. 2009;481:75-82. doi: 10.1007/978-1-59745-201-4_7. Methods Mol Biol. 2009. PMID: 19096792 Review.
Cited by
-
Producing Human Livers From Human Stem Cells Via Blastocyst Complementation.Curr Opin Biomed Eng. 2024 Sep;31:100537. doi: 10.1016/j.cobme.2024.100537. Epub 2024 May 16. Curr Opin Biomed Eng. 2024. PMID: 38854436
-
Generation of RAG2 Knockout Immune-Deficient Miniature Pigs.Animals (Basel). 2024 Sep 6;14(17):2597. doi: 10.3390/ani14172597. Animals (Basel). 2024. PMID: 39272382 Free PMC article.
-
Genetic engineering drives the breakthrough of pig models in liver disease research.Liver Res. 2024 Sep 16;8(3):131-140. doi: 10.1016/j.livres.2024.09.003. eCollection 2024 Sep. Liver Res. 2024. PMID: 39957748 Free PMC article. Review.
-
Immunometabolism of Liver Xenotransplantation and Prospective Solutions.Adv Sci (Weinh). 2025 Mar;12(9):e2407610. doi: 10.1002/advs.202407610. Epub 2025 Feb 6. Adv Sci (Weinh). 2025. PMID: 39912334 Free PMC article. Review.
-
Emerging and potential use of CRISPR in human liver disease.Hepatology. 2025 Jul 1;82(1):232-253. doi: 10.1097/HEP.0000000000000578. Epub 2025 Jun 19. Hepatology. 2025. PMID: 37607734 Review.
References
-
- Fisher RA, Strom SC. Human hepatocyte transplantation: worldwide results. Transplantation (2006) 82(4):441–9. - PubMed
-
- Chen AX, Chhabra A, Fleming HE, Bhatia SN. Chapter 40 - hepatic tissue engineering. In: Lanza R, Langer R, Atala JPV & A., editors. Principles of tissue engineering, Fifth Edition. London, United Kingdom: Academic Press; (2020). doi: 10.1016/B978-0-12-818422-6.00041-1 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

