Prenatal antibiotics exposure does not influence experimental allergic asthma in mice
- PMID: 36032166
- PMCID: PMC9399857
- DOI: 10.3389/fimmu.2022.937577
Prenatal antibiotics exposure does not influence experimental allergic asthma in mice
Abstract
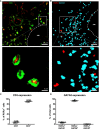
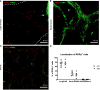
Changes in microbiome (dysbiosis) contribute to severity of allergic asthma. Preexisting epidemiological studies in humans correlate perinatal dysbiosis with increased long-term asthma severity. However, these studies cannot discriminate between prenatal and postnatal effects of dysbiosis and suffer from a high variability of dysbiotic causes ranging from antibiotic treatment, delivery by caesarian section to early-life breastfeeding practices. Given that maternal antibiotic exposure in mice increases the risk of newborn bacterial pneumonia in offspring, we hypothesized that prenatal maternal antibiotic-induced dysbiosis induces long-term immunological effects in the offspring that also increase long-term asthma severity. Therefore, dams were exposed to antibiotics (gentamycin, ampicillin, vancomycin) from embryonic day 15 until birth. Six weeks later, asthma was induced in the offspring by repeated applications of house dust mite extract. Airway function, cytokine production, pulmonary cell composition and distribution were assessed. Our study revealed that prenatally induced dysbiosis in mice led to an increase in pulmonary Th17+ non-conventional T cells with limited functional effect on airway resistance, pro-asthmatic Th2/Th17 cytokine production, pulmonary localization and cell-cell contacts. These data indicate that dysbiosis-related immune-modulation with long-term effects on asthma development occurs to a lesser extent prenatally and will allow to focus future studies on more decisive postnatal timeframes.
Keywords: ILCs; RORγt; Th17 response; asthma; dysbiosis; microbiome.
Copyright © 2022 Lingel, Wilburn, Hargis, McAlees, Laumonnier, Chougnet, Deshmukh, König, Lewkowich and Schmudde.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures