Mast cells and tryptase are linked to itch and disease severity in mycosis fungoides: Results of a pilot study
- PMID: 36032167
- PMCID: PMC9400509
- DOI: 10.3389/fimmu.2022.930979
Mast cells and tryptase are linked to itch and disease severity in mycosis fungoides: Results of a pilot study
Abstract
Introduction: In mycosis fungoides (MF), the most common cutaneous T-cell lymphoma, itch is a frequent clinical symptom. Whether mast cells (MCs), eosinophils (Eos) or their mediators play a role in MF-associated itch or disease severity is controversially discussed. Here, we explored the role of MC and Eo numbers in the skin as well as blood levels of their mediators in disease severity and itch.
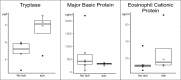
Methods: In 10 patients with MF and 10 matched control subjects we assessed disease severity, itch, and quality of life impairment using dedicated tools such as the mSWAT, ItchyQoL and DLQI. We analyzed skin biopsies and measured serum levels of tryptase, a mast cell mediator, as well as of the eosinophil products eosinophil cationic protein (ECP) and major basic protein (MBP).
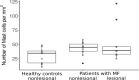
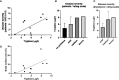
Results: The presence of chronic itch, in four of 10 patients, was associated with significantly higher disease severity (mSwat), larger body surface area affected, and stronger QoL impairment (Itchy-Qol, DLQI). Serum levels of tryptase, but not ECP and MBP, were linked with patient-reported disease severity, body surface area affected, and the presence of itch. Three of the four patients with chronic itch, but none of the six patients without, had tryptase levels above >6µg/l. Numbers of MCs in the papillary dermis were higher in MF skin lesions then in non-lesional skin of MF patients and skin of healthy controls.
Discussion: The MC-mediator tryptase, in MF, is linked to disease activity and impact, most prominently to itch. Our findings call for larger studies that explore the role of MCs, tryptase and other MC mediators as drivers of itch and their role in MF pathogenesis.
Keywords: cutaneous T-cell lymphoma; eosinophil; itch (pruritus); mast cell; mycosis fungoides; tryptase.
Copyright © 2022 Terhorst-Molawi, Lohse, Ginter, Puhl, Metz, Hu, Maurer and Altrichter.
Conflict of interest statement
DT-M has received research funds and was advisor for Celldex, Novartis, Sanofi and Moxie. MMe is or recently was a speaker and/or advisor for AbbVie, Amgen, ArgenX, AstraZeneca, Bayer, Celldex, Celgene, Escient, Galderma, Grünenthal, GSK, Menlo, Novartis, Pfizer, Pharvaris, Roche, Sanofi-Aventis, Third Harmonic Bio. MMa is or recently was a speaker and/or advisor for and/or has received research funding from Allakos, Aralez, Genentech, GSK, Menarini, Merckle Recordati, Moxie, Novartis, Sanofi, MSD, and Uriach. SA has conducted studies for received research funds/was advisor for Allakos, ALK, AstraZeneca, CSL Behring, LeoPharma, Moxie, Novartis, Sanofi, Takeda, Thermofisher. Published results are part of the study ROBERTIS, funded by AstraZeneca. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Dermatologists B. Mycosis fungoides - patient information leaflet. (2019). Available at: https://www.bad.org.uk/pils/mycosis-fungoides/ (Accessed 30th July 2022).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

