Pharmacokinetics and bioequivalence of two cyclosporine oral solution formulations in cats
- PMID: 36032284
- PMCID: PMC9399922
- DOI: 10.3389/fvets.2022.940472
Pharmacokinetics and bioequivalence of two cyclosporine oral solution formulations in cats
Abstract
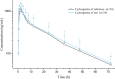
The pharmacokinetic profiles and bioequivalence of two cyclosporine oral solutions were investigated in cats. Twenty-four cats were randomly allocated to two equally sized treatment groups in a randomized four-cycle, and dual-sequence cross-over design. Test and reference articles were orally administered in a single dose of 7 mg/kg Bodyweight. Serial blood samples were collected, and blood cyclosporine concentration was determined by ultra-performance liquid chromatography-mass spectrometry (UPLC-MS/MS). No significant differences were present in the major pharmacokinetic parameters (Cmax, AUC0-last,) between the two formulations. The blood profiles of cyclosporine following the administration of both formulations were similar. The findings of the study suggested that the two articles were bioequivalent for cyclosporine oral solution.
Keywords: bioequivalence; cat; cyclosporine oral solution; four-cycle; pharmacokinetics.
Copyright © 2022 Yang, Kong, Liu, Wu, Cao, Qiu, Zhang, Gong, Zhao, Cao and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous