Investigation of the added value of CT-based radiomics in predicting the development of brain metastases in patients with radically treated stage III NSCLC
- PMID: 36032350
- PMCID: PMC9403451
- DOI: 10.1177/17588359221116605
Investigation of the added value of CT-based radiomics in predicting the development of brain metastases in patients with radically treated stage III NSCLC
Abstract
Introduction: Despite radical intent therapy for patients with stage III non-small-cell lung cancer (NSCLC), cumulative incidence of brain metastases (BM) reaches 30%. Current risk stratification methods fail to accurately identify these patients. As radiomics features have been shown to have predictive value, this study aims to develop a model combining clinical risk factors with radiomics features for BM development in patients with radically treated stage III NSCLC.
Methods: Retrospective analysis of two prospective multicentre studies. Inclusion criteria: adequately staged [18F-fluorodeoxyglucose positron emission tomography-computed tomography (18-FDG-PET-CT), contrast-enhanced chest CT, contrast-enhanced brain magnetic resonance imaging/CT] and radically treated stage III NSCLC, exclusion criteria: second primary within 2 years of NSCLC diagnosis and prior prophylactic cranial irradiation. Primary endpoint was BM development any time during follow-up (FU). CT-based radiomics features (N = 530) were extracted from the primary lung tumour on 18-FDG-PET-CT images, and a list of clinical features (N = 8) was collected. Univariate feature selection based on the area under the curve (AUC) of the receiver operating characteristic was performed to identify relevant features. Generalized linear models were trained using the selected features, and multivariate predictive performance was assessed through the AUC.
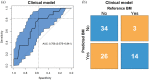
Results: In total, 219 patients were eligible for analysis. Median FU was 59.4 months for the training cohort and 67.3 months for the validation cohort; 21 (15%) and 17 (22%) patients developed BM in the training and validation cohort, respectively. Two relevant clinical features (age and adenocarcinoma histology) and four relevant radiomics features were identified as predictive. The clinical model yielded the highest AUC value of 0.71 (95% CI: 0.58-0.84), better than radiomics or a combination of clinical parameters and radiomics (both an AUC of 0.62, 95% CIs of 0.47-076 and 0.48-0.76, respectively).
Conclusion: CT-based radiomics features of primary NSCLC in the current setup could not improve on a model based on clinical predictors (age and adenocarcinoma histology) of BM development in radically treated stage III NSCLC patients.
Keywords: CT; metastatic brain tumours; non-small-cell lung cancer; predictive biomarker; tumour biology.
© The Author(s), 2022.
Conflict of interest statement
Competing interests: P.L. reports, within and outside the submitted work, grants/sponsored research agreements from Radiomics SA, ptTheragnostic/DNAmito, Health Innovation Ventures. He received an advisor/presenter fee and/or reimbursement of travel costs/consultancy fee and/or in kind manpower contribution from Radiomics SA, BHV, Merck, Varian, Elekta, ptTheragnostic, BMS, and Convert pharmaceuticals. Dr Lambin has minority shares in the company Radiomics SA, Convert pharmaceuticals, Comunicare Solutions, and LivingMed Biotech, he is a co-inventor of two issued patents with royalties on radiomics (PCT/NL2014/050248, PCT/NL2014/050728) licensed to Radiomics SA and one issued patent on mtDNA (PCT/EP2014/059089) licensed to ptTheragnostic/DNAmito, one non-issued patent on LSRT (PCT/ P126537PC00) licensed to Varian Medical, three non-patented invention (softwares) licensed to ptTheragnostic/DNAmito, Radiomics SA and Health Innovation Ventures and three non-issues, non-licensed patents on Deep & handcrafted Radiomics (US P125078US00, PCT/NL/2020/050794, n° N2028271). He confirms that none of the above entities or funding was involved in the preparation of this paper. LH: none related to current manuscript, outside of current manuscript: research funding Roche Genentech, Boehringer Ingelheim, AstraZeneca (all institution, furthermore Takeda and Beigene in negotiation [institution]); advisory board: BMS, Eli Lilly, Roche Genentech, Pfizer, Takeda, MSD, Boehringer Ingelheim, Amgen, Janssen (all institution, Roche one time self); speaker: MSD (institution); travel/conference reimbursement: Roche Genentech (self); mentorship program with key opinion leaders: funded by AstraZeneca; fees for educational webinars: Benecke, Medtalks, VJOncology (self), high5oncology (institution); interview sessions funded by Roche Genentech, Bayer (institution); local PI of clinical trials: AstraZeneca, Novartis, BMS, MSD /Merck, GSK, Takeda, Blueprint Medicines, Roche Genentech, Janssen Pharmaceuticals, Mirati. DdR: none related to current manuscript, outside of current manuscript: grants from BMS, AstraZeneca, Seat tle Genetics, Philips, Olink, BeiGene. Advisory board (no personal fees: AstraZeneca, Philips. HW: has minority shares in the company Radiomics SA. H.G: Advisory board (no personal fees): Roche, Astra-Zeneca, Boehringer-Ingelheim, Lilly, Novartis.
Figures


References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017; 67: 7–30. - PubMed
-
- Hendriks LE, Brouns AJ, Amini M, et al. Development of symptomatic brain metastases after chemoradiotherapy for stage III non-small cell lung cancer: does the type of chemotherapy regimen matter? Lung Cancer 2016; 101: 68–75. - PubMed
-
- Govindan R, Bogart J, Vokes EE. Locally advanced non-small cell lung cancer: the past, present, and future. J Thorac Oncol 2008; 3: 917–928. - PubMed
-
- Eberhardt WE, De Ruysscher D, Weder W, et al. 2nd ESMO Consensus Conference in Lung Cancer: locally advanced stage III non-small-cell lung cancer. Ann Oncol 2015; 26: 1573–1588. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

