Self-assembling short immunostimulatory duplex RNAs with broad-spectrum antiviral activity
- PMID: 36032397
- PMCID: PMC9398551
- DOI: 10.1016/j.omtn.2022.08.031
Self-assembling short immunostimulatory duplex RNAs with broad-spectrum antiviral activity
Abstract
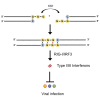
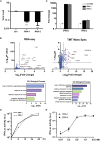
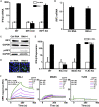
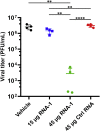
The current coronavirus disease 2019 (COVID-19) pandemic highlights the need for broad-spectrum antiviral therapeutics. Here we describe a new class of self-assembling immunostimulatory short duplex RNAs that potently induce production of type I and type III interferon (IFN-I and IFN-III). These RNAs require a minimum of 20 base pairs, lack any sequence or structural characteristics of known immunostimulatory RNAs, and instead require a unique sequence motif (sense strand, 5'-C; antisense strand, 3'-GGG) that mediates end-to-end dimer self-assembly. The presence of terminal hydroxyl or monophosphate groups, blunt or overhanging ends, or terminal RNA or DNA bases did not affect their ability to induce IFN. Unlike previously described immunostimulatory small interfering RNAs (siRNAs), their activity is independent of Toll-like receptor (TLR) 7/8, but requires the RIG-I/IRF3 pathway that induces a more restricted antiviral response with a lower proinflammatory signature compared with immunostimulant poly(I:C). Immune stimulation mediated by these duplex RNAs results in broad-spectrum inhibition of infections by many respiratory viruses with pandemic potential, including severe acute respiratory syndrome coronavirus (SARS-CoV)-2, SARS-CoV, Middle East respiratory syndrome coronavirus (MERS-CoV), human coronavirus (HCoV)-NL63, and influenza A virus in cell lines, human lung chips that mimic organ-level lung pathophysiology, and a mouse SARS-CoV-2 infection model. These short double-stranded RNAs (dsRNAs) can be manufactured easily, and thus potentially could be harnessed to produce broad-spectrum antiviral therapeutics.
Keywords: Hoogsteen G-G base pairing; MT: Oligonucleotides; RIG-I; SARS-CoV-2; Therapies and Applications; antiviral therapeutic; immunostimulatory RNA; influenza; interferon; organ on a chip.
© 2022 The Author(s).
Conflict of interest statement
D.E.I. is a founder, board member, SAB chair, and equity holder in Emulate Inc. D.E.I., L.S., H.B., C.O., and R.P. are inventors on relevant patent applications held by Harvard University.
Figures
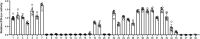


Update of
-
Self-assembling short immunostimulatory duplex RNAs with broad spectrum antiviral activity.bioRxiv [Preprint]. 2021 Nov 22:2021.11.19.469183. doi: 10.1101/2021.11.19.469183. bioRxiv. 2021. Update in: Mol Ther Nucleic Acids. 2022 Sep 13;29:923-940. doi: 10.1016/j.omtn.2022.08.031. PMID: 34845453 Free PMC article. Updated. Preprint.
References
-
- Ren X., Linehan M.M., Iwasaki A., Pyle A.M. RIG-I Selectively Discriminates against 5'-monophosphate RNA. Cell Rep. 2019;26:2019–2027.e4. - PubMed
-
- Ren X., Linehan M.M., Iwasaki A., Pyle A.M. RIG-I recognition of RNA targets: the influence of terminal base pair sequence and overhangs on affinity and signaling. Cell Rep. 2019;29:3807–3815.e3. - PubMed
-
- Marques J.T., Williams B.R.G. Activation of the mammalian immune system by siRNAs. Nat. Biotechnol. 2005;23:1399–1405. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

