Designing Microbial Cell Factories for the Production of Chemicals
- PMID: 36032533
- PMCID: PMC9400054
- DOI: 10.1021/jacsau.2c00344
Designing Microbial Cell Factories for the Production of Chemicals
Abstract
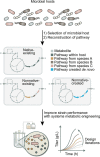
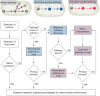
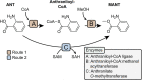
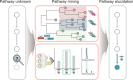
The sustainable production of chemicals from renewable, nonedible biomass has emerged as an essential alternative to address pressing environmental issues arising from our heavy dependence on fossil resources. Microbial cell factories are engineered microorganisms harboring biosynthetic pathways streamlined to produce chemicals of interests from renewable carbon sources. The biosynthetic pathways for the production of chemicals can be defined into three categories with reference to the microbial host selected for engineering: native-existing pathways, nonnative-existing pathways, and nonnative-created pathways. Recent trends in leveraging native-existing pathways, discovering nonnative-existing pathways, and designing de novo pathways (as nonnative-created pathways) are discussed in this Perspective. We highlight key approaches and successful case studies that exemplify these concepts. Once these pathways are designed and constructed in the microbial cell factory, systems metabolic engineering strategies can be used to improve the performance of the strain to meet industrial production standards. In the second part of the Perspective, current trends in design tools and strategies for systems metabolic engineering are discussed with an eye toward the future. Finally, we survey current and future challenges that need to be addressed to advance microbial cell factories for the sustainable production of chemicals.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
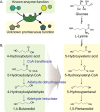
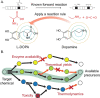
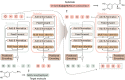

References
-
- Lee S. Y.; Kim H. U.; Chae T. U.; Cho J. S.; Kim J. W.; Shin J. H.; Kim D. I.; Ko Y.-S.; Jang W. D.; Jang Y.-S. A comprehensive metabolic map for production of bio-based chemicals. Nat. Catal. 2019, 2 (1), 18–33. 10.1038/s41929-018-0212-4. - DOI
Publication types
LinkOut - more resources
Full Text Sources
