Ultrathin, high-speed, all-optical photoacoustic endomicroscopy probe for guiding minimally invasive surgery
- PMID: 36032566
- PMCID: PMC9408236
- DOI: 10.1364/BOE.463057
Ultrathin, high-speed, all-optical photoacoustic endomicroscopy probe for guiding minimally invasive surgery
Abstract
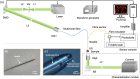
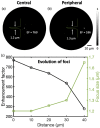
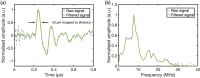
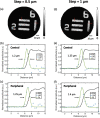
Photoacoustic (PA) endoscopy has shown significant potential for clinical diagnosis and surgical guidance. Multimode fibres (MMFs) are becoming increasingly attractive for the development of miniature endoscopy probes owing to their ultrathin size, low cost and diffraction-limited spatial resolution enabled by wavefront shaping. However, current MMF-based PA endomicroscopy probes are either limited by a bulky ultrasound detector or a low imaging speed that hindered their usability. In this work, we report the development of a highly miniaturised and high-speed PA endomicroscopy probe that is integrated within the cannula of a 20 gauge medical needle. This probe comprises a MMF for delivering the PA excitation light and a single-mode optical fibre with a plano-concave microresonator for ultrasound detection. Wavefront shaping with a digital micromirror device enabled rapid raster-scanning of a focused light spot at the distal end of the MMF for tissue interrogation. High-resolution PA imaging of mouse red blood cells covering an area 100 µm in diameter was achieved with the needle probe at ∼3 frames per second. Mosaicing imaging was performed after fibre characterisation by translating the needle probe to enlarge the field-of-view in real-time. The developed ultrathin PA endomicroscopy probe is promising for guiding minimally invasive surgery by providing functional, molecular and microstructural information of tissue in real-time.
Published by Optica Publishing Group under the terms of the Creative Commons Attribution 4.0 License. Further distribution of this work must maintain attribution to the author(s) and the published article’s title, journal citation, and DOI.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships. T.V. is co-founder and shareholder of Hypervision Surgical Ltd, London, UK. He is also a shareholder of Mauna Kea Technologies, Paris, France. P.B. and E.Z. are shareholders in DeepColor Imaging SAS.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
