Tight junction disruption through activation of the PI3K/AKT pathways in the skin contributes to blister fluid formation after severe tibial plateau fracture
- PMID: 36032734
- PMCID: PMC9403793
- DOI: 10.3389/fbioe.2022.946261
Tight junction disruption through activation of the PI3K/AKT pathways in the skin contributes to blister fluid formation after severe tibial plateau fracture
Abstract
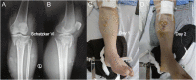
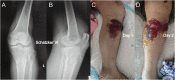
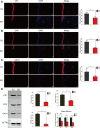
Background: Acute compartment syndrome (ACS) is an orthopedic emergency that commonly occurs after severe tibial plateau fracture. Fracture blisters form on the skin, and it was found in our previous study that when blisters form, the compartment pressure significantly decreases. However, the potential mechanism underlying this pressure decrease has not yet been elucidated. Methods: To obtain a comprehensive understanding of the changes that occur after blister formation on the skin, the changes in tight junction expression in the skin after tibial plateau fracture were observed. Blister samples and normal skin were collected from patients with bicondylar tibial plateau fractures with or without blisters. The epidermis thickness was measured, and the difference in the levels of K1, K5, K10, and skin barrier proteins such as claudin 1, claudin 2, and occludin between the two groups was evaluated by immunochemistry analysis, immunofluorescence, Western blotting, and qPCR. Results: The skin was thinner and the levels of K1, K5, and K10 were significantly decreased in blistered skin. Furthermore, the PI3K/AKT pathway was found to be activated, and the tight junction expression was significantly decreased in blistered skin. This indicates that the paracellular pathway, which is essential for accelerating fluid accumulation in blisters and indirectly decreases compartment pressure, was activated. Conclusion: Changes in the tight junction expression after blister formation may underlie blister fluid formation and indirectly explain the decrease in compartment pressure under blistered skin after severe tibial plateau fracture.
Keywords: acute compartment syndrome; fracture blisters; paracellular pathway; tibial plateau fracture; tight junctions.
Copyright © 2022 Guo, Chen, Lin, Jin, Hou, Dong and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




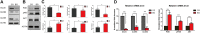
References
LinkOut - more resources
Full Text Sources
Research Materials

