Intersubunit Coupling Enables Fast CO2-Fixation by Reductive Carboxylases
- PMID: 36032767
- PMCID: PMC9413435
- DOI: 10.1021/acscentsci.2c00057
Intersubunit Coupling Enables Fast CO2-Fixation by Reductive Carboxylases
Abstract
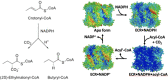
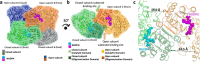
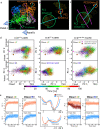
Enoyl-CoA carboxylases/reductases (ECRs) are some of the most efficient CO2-fixing enzymes described to date. However, the molecular mechanisms underlying the extraordinary catalytic activity of ECRs on the level of the protein assembly remain elusive. Here we used a combination of ambient-temperature X-ray free electron laser (XFEL) and cryogenic synchrotron experiments to study the structural organization of the ECR from Kitasatospora setae. The K. setae ECR is a homotetramer that differentiates into a pair of dimers of open- and closed-form subunits in the catalytically active state. Using molecular dynamics simulations and structure-based mutagenesis, we show that catalysis is synchronized in the K. setae ECR across the pair of dimers. This conformational coupling of catalytic domains is conferred by individual amino acids to achieve high CO2-fixation rates. Our results provide unprecedented insights into the dynamic organization and synchronized inter- and intrasubunit communications of this remarkably efficient CO2-fixing enzyme during catalysis.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Amao Y. Formate dehydrogenase for CO2 utilization and its application. J. of CO22 Utilization 2018, 26, 623–641. 10.1016/j.jcou.2018.06.022. - DOI

