Locally delivered antistaphylococcal lysin exebacase or CF-296 is active in methicillin-resistant Staphylococcus aureus implant-associated osteomyelitis
- PMID: 36032801
- PMCID: PMC9399932
- DOI: 10.5194/jbji-7-169-2022
Locally delivered antistaphylococcal lysin exebacase or CF-296 is active in methicillin-resistant Staphylococcus aureus implant-associated osteomyelitis
Abstract
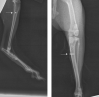
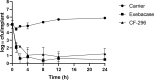
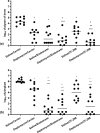
Introduction: Staphylococcus aureus is the most common cause of orthopedic infections and can be challenging to treat, especially in the presence of a foreign body. The antistaphylococcal lysins exebacase and CF-296 have rapid bactericidal activity, a low propensity for resistance development, and synergize with some antibiotics. Methods: Rabbit implant-associated osteomyelitis was induced by drilling into the medial tibia followed by locally delivering exebacase, CF-296, or lysin carrier. A titanium screw colonized with methicillin-resistant S. aureus (MRSA) IDRL-6169 was inserted. Intravenous daptomycin or saline was administered and continued daily for 4 d. On day 5, rabbits were euthanized, and the tibiae and implants were collected for culture. Results were reported as log colony forming units (cfu) per gram of bone or log cfu per implant, and comparisons among the six groups were performed using the Wilcoxon rank sum test. Results: Based on implant and bone cultures, all treatments resulted in significantly lower bacterial counts than those of controls ( ). Exebacase alone or with daptomycin as well as CF-296 with daptomycin were more active than daptomycin alone ( ) or CF-296 alone ( ) based on implant cultures. CF-296 with daptomycin was more active than either CF-296 alone ( ) or daptomycin alone ( ) based on bone cultures. Conclusion: Local delivery of either exebacase or CF-296 offers a promising complement to conventional antibiotics in implant-associated infections.
Copyright: © 2022 Melissa Karau et al.
Conflict of interest statement
Robin Patel declares financial support (grants) from ContraFect Corporation, TenNor Therapeutics Limited, and BioFire Diagnostics. Robin Patel is a consultant to Curetis, Next Gen Diagnostics, PathoQuest, Selux Diagnostics, 1928 Diagnostics, PhAST, Torus Biosystems, Day Zero Diagnostics, Mammoth Biosciences, and Qvella Corporation; monies are paid to Mayo Clinic. Mayo Clinic and Robin Patel have a relationship with Pathogenomix. Robin Patel has research supported by Adaptive Phage Therapeutics. Mayo Clinic has a royalty-bearing know-how agreement and equity in Adaptive Phage Therapeutics. Robin Patel is also a consultant to Netflix and CARB-X. In addition, Robin Patel has a patent on PCR issued, a patent on a device/method for sonication with royalties paid by Samsung to Mayo Clinic, and a patent on an anti-biofilm substance issued. Robin Patel receives honoraria from the NBME, Up-to-Date, and the Infectious Diseases Board Review Course.
Figures


Similar articles
-
In vitro activity of exebacase against methicillin-resistant Staphylococcus aureus biofilms on orthopedic Kirschner wires.BMC Res Notes. 2023 Sep 11;16(1):209. doi: 10.1186/s13104-023-06468-y. BMC Res Notes. 2023. PMID: 37697424 Free PMC article.
-
Exebacase Is Active In Vitro in Pulmonary Surfactant and Is Efficacious Alone and Synergistic with Daptomycin in a Mouse Model of Lethal Staphylococcus aureus Lung Infection.Antimicrob Agents Chemother. 2021 Aug 17;65(9):e0272320. doi: 10.1128/AAC.02723-20. Epub 2021 Aug 17. Antimicrob Agents Chemother. 2021. PMID: 34228536 Free PMC article.
-
Exebacase in Addition to Daptomycin Is More Active than Daptomycin or Exebacase Alone in Methicillin-Resistant Staphylococcus aureus Osteomyelitis in Rats.Antimicrob Agents Chemother. 2019 Sep 23;63(10):e01235-19. doi: 10.1128/AAC.01235-19. Print 2019 Oct. Antimicrob Agents Chemother. 2019. PMID: 31358593 Free PMC article.
-
Exebacase: A Novel Approach to the Treatment of Staphylococcal Infections.Drugs R D. 2022 Jun;22(2):113-117. doi: 10.1007/s40268-022-00383-6. Epub 2022 Feb 17. Drugs R D. 2022. PMID: 35175568 Free PMC article. Review.
-
Daptomycin in bone and joint infections: a review of the literature.Arch Orthop Trauma Surg. 2009 Nov;129(11):1495-504. doi: 10.1007/s00402-008-0772-x. Epub 2008 Nov 7. Arch Orthop Trauma Surg. 2009. PMID: 18989686 Free PMC article. Review.
Cited by
-
Rapid bacteriolysis of Staphylococcus aureus by lysin exebacase.Microbiol Spectr. 2023 Aug 10;11(5):e0190623. doi: 10.1128/spectrum.01906-23. Online ahead of print. Microbiol Spectr. 2023. PMID: 37561151 Free PMC article.
-
Cost-effectiveness of a bone substitute delivering gentamicin in the treatment of chronic osteomyelitis of long bones: Protocol for the CONVICTION randomized multicenter study.Front Med (Lausanne). 2023 Mar 30;10:1116711. doi: 10.3389/fmed.2023.1116711. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37064036 Free PMC article.
-
Exploring the potential of naturally occurring antimicrobials for managing orthopedic-device-related infections.J Bone Jt Infect. 2024 Oct 31;9(5):249-260. doi: 10.5194/jbji-9-249-2024. eCollection 2024. J Bone Jt Infect. 2024. PMID: 39539734 Free PMC article. Review.
-
In vitro activity of exebacase against methicillin-resistant Staphylococcus aureus biofilms on orthopedic Kirschner wires.BMC Res Notes. 2023 Sep 11;16(1):209. doi: 10.1186/s13104-023-06468-y. BMC Res Notes. 2023. PMID: 37697424 Free PMC article.
-
Bacterial persisters: molecular mechanisms and therapeutic development.Signal Transduct Target Ther. 2024 Jul 17;9(1):174. doi: 10.1038/s41392-024-01866-5. Signal Transduct Target Ther. 2024. PMID: 39013893 Free PMC article. Review.
References
-
- Alder KD, Lee I, Munger AM, Kwon H-K, Morris MT, Cahill SV, Back J, Kristin EY, Lee FY. Intracellular Staphylococcus aureus in bone and joint infections: A mechanism of disease recurrence, inflammation, and bone and cartilage destruction. Bone. 2020;141:115568. doi: 10.1016/j.bone.2020.115568. - DOI - PubMed
-
- Direct lysis of resistant pathogen trial of exebacase (DISRUPT), NCT04160468 [Internet] Clinicaltrials.gov; [last access: 24 September 2021]. 2021. http://clinicaltrials.gov.
LinkOut - more resources
Full Text Sources