This is a preprint.
Rapidly shifting immunologic landscape and severity of SARS-CoV-2 in the Omicron era in South Africa
- PMID: 36032973
- PMCID: PMC9413704
- DOI: 10.1101/2022.08.19.22278993
Rapidly shifting immunologic landscape and severity of SARS-CoV-2 in the Omicron era in South Africa
Update in
-
Rapidly shifting immunologic landscape and severity of SARS-CoV-2 in the Omicron era in South Africa.Nat Commun. 2023 Jan 16;14(1):246. doi: 10.1038/s41467-022-35652-0. Nat Commun. 2023. PMID: 36646700 Free PMC article.
Abstract
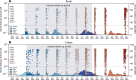
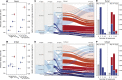
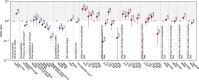
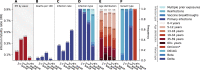
South Africa was among the first countries to detect the SARS-CoV-2 Omicron variant. Propelled by increased transmissibility and immune escape properties, Omicron displaced other globally circulating variants within 3 months of its emergence. Due to limited testing, Omicron's attenuated clinical severity, and an increased risk of reinfection, the size of the Omicron BA.1 and BA.2 subvariants (BA.1/2) wave remains poorly understood in South Africa and in many other countries. Using South African data from urban and rural cohorts closely monitored since the beginning of the pandemic, we analyzed sequential serum samples collected before, during, and after the Omicron BA.1/2 wave to infer infection rates and monitor changes in the immune histories of participants over time. Omicron BA.1/2 infection attack rates reached 65% (95% CI, 60% - 69%) in the rural cohort and 58% (95% CI, 61% - 74%) in the urban cohort, with repeat infections and vaccine breakthroughs accounting for >60% of all infections at both sites. Combined with previously collected data on pre-Omicron variant infections within the same cohorts, we identified 14 distinct categories of SARS-CoV-2 antigen exposure histories in the aftermath of the Omicron BA.1/2 wave, indicating a particularly fragmented immunologic landscape. Few individuals (<6%) remained naïve to SARS-CoV-2 and no exposure history category represented over 25% of the population at either cohort site. Further, cohort participants were more than twice as likely to get infected during the Omicron BA.1/2 wave, compared to the Delta wave. Prior infection with the ancestral strain (with D614G mutation), Beta, and Delta variants provided 13% (95% CI, -21% - 37%), 34% (95% CI, 17% - 48%), and 51% (95% CI, 39% - 60%) protection against Omicron BA.1/2 infection, respectively. Hybrid immunity (prior infection and vaccination) and repeated prior infections (without vaccination) reduced the risks of Omicron BA.1/2 infection by 60% (95% CI, 42% - 72%) and 85% (95% CI, 76% - 92%) respectively. Reinfections and vaccine breakthroughs had 41% (95% CI, 26% - 53%) lower risk of onward transmission than primary infections. Our study sheds light on a rapidly shifting landscape of population immunity, along with the changing characteristics of SARS-CoV-2, and how these factors interact to shape the success of emerging variants. Our findings are especially relevant to populations similar to South Africa with low SARS-CoV-2 vaccine coverage and a dominant contribution of immunity from prior infection. Looking forward, the study provides context for anticipating the long-term circulation of SARS-CoV-2 in populations no longer naïve to the virus.
Conflict of interest statement
Figures
References
-
- Tracking SARS-CoV-2 variants, (available at https://www.who.int/activities/tracking-SARS-CoV-2-variants).
-
- CoVariants, (available at https://covariants.org/).
-
- Obermeyer F., Jankowiak M., Barkas N., Schaffner S. F., Pyle J. D., Yurkovetskiy L., Bosso M., Park D. J., Babadi M., MacInnis B. L., Luban J., Sabeti P. C., Lemieux J. E., Analysis of 6.4 million SARS-CoV-2 genomes identifies mutations associated with fitness. Science. 376, 1327–1332 (2022). - PMC - PubMed
-
- the Nextstrain team, Genomic epidemiology of novel coronavirus - Global subsampling. Nextstrain (2022) (available at https://nextstrain.org/ncov/gisaid/global).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
