A Real-World Study on the Effect of Imrecoxib for Patients with Axial Spondyloarthritis
- PMID: 36033132
- PMCID: PMC9416422
- DOI: 10.2147/DDDT.S376406
A Real-World Study on the Effect of Imrecoxib for Patients with Axial Spondyloarthritis
Abstract
Purpose: Non-steroidal anti-inflammatory drugs (NSAIDs) have generally been viewed as first-line therapy for axial spondyloarthritis (axSpA). Imrecoxib is a selective COX-2 inhibitor developed independently in China. At present, only one single-center RCT trial has shown that imrecoxib is equally effective as celecoxib in treating axSpA. Based on real-world data, our study aims to explore the efficiency of imrecoxib and TNF inhibitor (TNFi) combined with imrecoxib in treating axSpA.
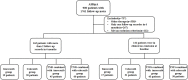
Patients and methods: A total of 163 patients with axSpA who had more than two follow-up records in 6 months and treated with imrecoxib/celecoxib/TNFi combined with imrecoxib/TNFi combined with celecoxib from the First Affiliated Hospital of Anhui Medical University SpA Real World Database (AHSpA) were selected for analysis of our study. The linear mixed model was used to compare efficacy indexes before and after treatment and between different groups, adjust baseline measurement value and follow-up time. The Kaplan-Meier survival analysis was used to identify the differences in cumulative clinical remission rates between groups with different treatment at the follow-up period.
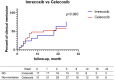
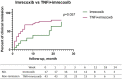
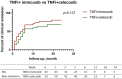
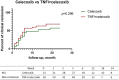
Results: Results showed that after treatment ASDAScrp was slightly improved in imrecoxib group and celecoxib group within 6 months (p < 0.05). CRP, ESR, BASDAI, ASDAScrp, BASFI, occiput to wall distance and finger floor distance all significantly improved in TNFi combined with imrecoxib group and TNFi combined with celecoxib group within 6 months (all p < 0.05). According to the Kaplan-Meier survival curve and Log rank test analysis, the clinical remission rate was not significantly different between different treatment during 24-month follow-up (all p > 0.05).
Conclusion: ASDAScrp improved slightly within 6 months after treatment with imrecoxib, and TNFi combined with imrecoxib significantly improved multiple effect indexes in axSpA patients. The efficacy of imrecoxib and celecoxib in the treatment of axSpA is equivalent. Also, they have the same efficacy after being combined with TNFi.
Keywords: TNF inhibitor; axial spondyloarthritis; celecoxib; imrecoxib.
© 2022 Zong et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

References
-
- Bohn R, Cooney M, Deodhar A, Curtis JR, Golembesky A. Incidence and prevalence of axial spondyloarthritis: methodologic challenges and gaps in the literature. Clin Exp Rheumatol. 2018;36:263–274. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

