Cobalt-Catalyzed Hydrogenation Reactions Enabled by Ligand-Based Storage of Dihydrogen
- PMID: 36033368
- PMCID: PMC9396622
- DOI: 10.1021/acscatal.2c02467
Cobalt-Catalyzed Hydrogenation Reactions Enabled by Ligand-Based Storage of Dihydrogen
Abstract
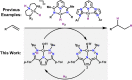
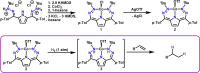
The use of supporting ligands that can store either protons or electrons has emerged as a powerful strategy in catalysis. While these strategies are potent individually, natural systems mediate remarkable transformations by combining the storage of both protons and electrons in the secondary coordination sphere. As such, there has been recent interest in using this strategy to enable fundamentally different transformations. Furthermore, outsourcing H-atom or hydrogen storage to ancillary ligands can also enable alternative mechanistic pathways and thereby selectivity. Here, we describe the application of this strategy to facilitate radical reactivity in Co-based hydrogenation catalysis. Metalation of previously reported dihydrazonopyrrole ligands with Co results in paramagnetic complexes, which are best described as having Co(II) oxidation states. These complexes catalytically hydrogenate olefins with low catalyst loadings under mild conditions (1 atm H2, 23 °C). Mechanistic, spectroscopic, and computational investigations indicate that this system goes through a radical hydrogen-atom transfer (HAT) type pathway that is distinct from classic organometallic mechanisms and is supported by the ability of the ligand to store H2. These results show how ancillary ligands can facilitate efficient catalysis, and furthermore how classic organometallic mechanisms for catalysis can be altered by the secondary coordination sphere.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Ito N.; Phillips S. E. V.; Stevens C.; Ogel Z. B.; McPherson M. J.; Keen J. N.; Yadav K. D. S.; Knowles P. F. Novel thioether bond revealed by a 1.7 Å crystal structure of galactose oxidase. Nature 1991, 350, 87–90. 10.1038/350087a0. - DOI - PubMed
- Ito N.; Phillips S. E. V.; Stevens C.; Ogel Z. B.; McPherson M. J.; Keen J. N.; Yadav K. D. S.; Knowles P. F. Three-dimensional structure of galactose oxidase: an enzyme with a built-in secondary cofactor. Faraday Discuss. 1992, 93, 75. 10.1039/fd9929300075. - DOI - PubMed
- Branchaud B. P.; Montagne-Smith M. P.; Kosman D. J.; McLaren F. R. Mechanism-based inactivation of galactose oxidase: evidence for a radical mechanism. J. Am. Chem. Soc. 1993, 115, 798. 10.1021/ja00055a067. - DOI
- Whittaker M. M.; Whittaker J. M. Ligand interactions with galactose oxidase: mechanistic insights. Biophys. J. 1993, 64, 762. 10.1016/S0006-3495(93)81437-1. - DOI - PMC - PubMed
- Ito N.; Phillips S. E. V.; Yadav K. D. S.; Knowles P. F. Crystal structure of a free radical enzyme, galactose oxidase. J. Mol. Biol. 1994, 238, 794. 10.1006/jmbi.1994.1335. - DOI - PubMed
- Knowles P. F.; Ito N.. Perspectives in Bio-inorganic Chemistry, Jai Press Ltd., 1994; Vol. 2, pp 207–244.
- Wachter R. M.; Branchaud B. P. Molecular modeling studies on oxidation of hexopyranoses by galactose oxidase. An active site topology apparently designed to catalyze radical reactions, either concerted or stepwise. J. Am. Chem. Soc. 1996, 118, 2782. 10.1021/ja9519896. - DOI
- Wachter R. M.; Montagne-Smith M. P.; Branchaud B. P. β-Haloethanol substrates as probes for radical mechanisms for galactose oxidase. J.Am. Chem. Soc. 1997, 119, 7743. 10.1021/ja9626695. - DOI
- Whittaker M. M.; Ballou D. P.; Whittaker J. W. Kinetic isotope effects as probes of the mechanism of galactose oxidase. Biochemistry 1998, 37, 8426. 10.1021/bi980328t. - DOI - PubMed
- Whittaker J. W.; Whittaker M. M. Radical copper oxidases, one electron at a time. Pure Appl. Chem. 1998, 70, 903. 10.1351/pac199870040903. - DOI
- Chaudhuri P.; Hess M.; Müller J.; Hildenbrand K.; Bill E.; Weyhermüller T.; Wieghardt K. Aerobic oxidation of primary alcohols (including methanol) by Copper (II)– and Zinc (II)– phenoxyl radical catalysts. J. Am. Chem. Soc. 1999, 121, 9599–9610. 10.1021/ja991481t. - DOI
- Cook S. A.; Hill E. A.; Borovik A. S. Lessons from nature: a bio-inspired approach to molecular design. Biochemistry 2015, 54, 4167–4180. 10.1021/acs.biochem.5b00249. - DOI - PMC - PubMed
- Baumgardner D. F.; Parks W. E.; Gilbertson J. D. Harnessing the active site triad: merging hemilability, proton responsivity, and ligand-based redox-activity. Dalton Trans. 2020, 49, 960–965. 10.1039/C9DT04470A. - DOI - PMC - PubMed
-
- Rakowski Dubois M.; Dubois D. L. Development of Molecular Electrocatalysts for CO2 Reduction and H2 Production/Oxidation. Acc. Chem. Res. 2009, 42, 1974–1982. 10.1021/ar900110c. - DOI - PubMed
- Zell T.; Milstein D. Hydrogenation and dehydrogenation iron pincer catalysts capable of metal–ligand cooperation by aromatization/dearomatization. Acc. Chem. Res. 2015, 48, 1979–1994. 10.1021/acs.accounts.5b00027. - DOI - PubMed
- Pegis M. L.; Wise C. F.; Martin D. J.; Mayer J. M. Oxygen reduction by homogeneous molecular catalysts and electrocatalysts. Chem. Rev. 2018, 118, 2340–2391. 10.1021/acs.chemrev.7b00542. - DOI - PubMed
-
- Luca O. R.; Crabtree R. H. Redox-active ligands in catalysis. Chem. Soc. Rev. 2013, 42, 1440–1459. 10.1039/C2CS35228A. - DOI - PubMed
- Arevalo R.; Chirik P. J. Enabling two-electron pathways with iron and cobalt: from ligand design to catalytic applications. J. Am. Chem. Soc. 2019, 141, 9106–9123. 10.1021/jacs.9b03337. - DOI - PMC - PubMed
- Ott J. C.; Bürgy D.; Guan H.; Gade L. H. 3d Metal Complexes in T-shaped Geometry as a Gateway to Metalloradical Reactivity. Acc. Chem. Res. 2022, 55, 857–868. 10.1021/acs.accounts.1c00737. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
