The All of Us Research Program: Data quality, utility, and diversity
- PMID: 36033590
- PMCID: PMC9403360
- DOI: 10.1016/j.patter.2022.100570
The All of Us Research Program: Data quality, utility, and diversity
Abstract
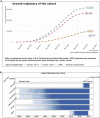
The All of Us Research Program seeks to engage at least one million diverse participants to advance precision medicine and improve human health. We describe here the cloud-based Researcher Workbench that uses a data passport model to democratize access to analytical tools and participant information including survey, physical measurement, and electronic health record (EHR) data. We also present validation study findings for several common complex diseases to demonstrate use of this novel platform in 315,000 participants, 78% of whom are from groups historically underrepresented in biomedical research, including 49% self-reporting non-White races. Replication findings include medication usage pattern differences by race in depression and type 2 diabetes, validation of known cancer associations with smoking, and calculation of cardiovascular risk scores by reported race effects. The cloud-based Researcher Workbench represents an important advance in enabling secure access for a broad range of researchers to this large resource and analytical tools.
Keywords: All of Us Research Program; cloud-based analytics; cohort study; electronic health records; precision medicine.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Devaney S. All of us. Nature. 2019;576:S14–S17. doi: 10.1038/d41586-019-03717-8. - DOI
-
- All of Us Research Hub . 2020. Researcher Workbench.https://www.researchallofus.org/workbench/

