Novel Antiamoebic Tyrocidine-Derived Peptide against Brain-Eating Amoebae
- PMID: 36033708
- PMCID: PMC9404165
- DOI: 10.1021/acsomega.2c01614
Novel Antiamoebic Tyrocidine-Derived Peptide against Brain-Eating Amoebae
Abstract
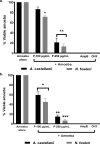
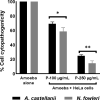
Acanthamoeba castellanii (A. castellanii) can cause Acanthamoeba keratitis, a sight-threatening infection, as well as a fatal brain infection termed granulomatous amoebic encephalitis, mostly in immunocompromised individuals. In contrast, Naegleria fowleri (N. fowleri) causes a deadly infection involving the central nervous system, recognized as primary amoebic encephalitis, mainly in individuals partaking in recreational water activities or those with nasal exposure to contaminated water. Worryingly, mortality rates due to these infections are more than 90%, suggesting the need to find alternative therapies. In this study, antiamoebic activity of a peptide based on the structure of the antibiotic tyrocidine was evaluated against A. castellanii and N. fowleri. The tyrocidine-derived peptide displayed significant amoebicidal efficacy against A. castellanii and N. fowleri. At 250 μg/mL, the peptide drastically reduced amoebae viability up to 13% and 21% after 2 h of incubation against N. fowleri and A. castellanii., whereas, after 24 h of incubation, the peptide showed 86% and 94% amoebicidal activity against A. castellanii and N. fowleri. Furthermore, amoebae pretreated with 100 μg/mL peptide inhibited 35% and 53% A. castellanii and N. fowleri, while, at 250 μg/mL, 84% and 94% A. castellanii and N. fowleri failed to adhere to human cells. Amoeba-mediated cell cytopathogenicity assays revealed 31% and 42% inhibition at 100 μg/mL, while at 250 μg/mL 75% and 86% A. castellanii and N. fowleri were inhibited. Assays revealed inhibition of encystation in both A. castellanii (58% and 93%) and N. fowleri (73% and 97%) at concentrations of 100 and 250 μg/mL respectively. Importantly, tyrocidine-derived peptide depicted minimal cytotoxicity to human cells and, thus, may be a potential candidate in the rational development of a treatment regimen against free-living amoebae infections. Future studies are necessary to elucidate the in vivo effects of tyrocidine-derived peptide against these and other pathogenic amoebae of importance.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Mungroo M. R.; Khan N. A.; Siddiqui R. Naegleria fowleri: diagnosis, treatment options and pathogenesis. Expert Opin. Orphan Drugs 2019, 7 (2), 67–80. 10.1080/21678707.2019.1571904. - DOI
LinkOut - more resources
Full Text Sources

