Metagenomic profiles of Dermacentor tick pathogens from across Mongolia, using next generation sequencing
- PMID: 36033893
- PMCID: PMC9399792
- DOI: 10.3389/fmicb.2022.946631
Metagenomic profiles of Dermacentor tick pathogens from across Mongolia, using next generation sequencing
Abstract
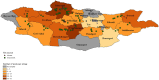
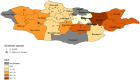
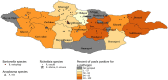
Tick-borne diseases are a major public health concern in Mongolia. Nomadic pastoralists, which make up ~ 26% of Mongolia's population, are at an increased risk of both tick bite exposure and economic loss associated with clinical disease in herds. This study sought to further characterize tick-borne pathogens present in Dermacentor ticks (n = 1,773) sampled in 2019 from 15 of Mongolia's 21 aimags (provinces). The ticks were morphologically identified and sorted into 377 pools which were then screened using Next-Generation Sequencing paired with confirmatory PCR and DNA sequence analysis. Rickettsia spp. were detected in 88.33% of pools, while Anaplasma spp. and Bartonella spp. were detected in 3.18 and 0.79% of pools, respectively. Khentii had the highest infection rate for Rickettsia spp. (76.61%; CI: 34.65-94.79%), while Arkhangai had the highest infection rate for Anaplasma spp. (7.79%; CI:4.04-13.72%). The exclusive detection of Anaplasma spp. in tick pools collected from livestock supports previous work in this area that suggests livestock play a significant role in disease maintenance. The detection of Anaplasma, Bartonella, and Rickettsia demonstrates a heightened risk for infection throughout Mongolia, with this study, to our knowledge, documenting the first detection of Bartonella melophagi in ticks collected in Mongolia. Further research deploying NGS methods is needed to characterize tick-borne pathogens in other endemic tick species found in Mongolia, including Hyalomma asiaticum and Ixodes persulcatus.
Keywords: Anaplasma; Bartonella; Dermacentor; Mongolia; Rickettsia; next generation sequencing; surveillance; tick-bome disease.
Copyright © 2022 Altantogtokh, Lilak, Takhampunya, Sakolvaree, Chanarat, Matulis, Poole-Smith, Boldbaatar, Davidson, Hertz, Bolorchimeg, Tsogbadrakh, Fiorenzano, Lindroth and von Fricken.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Barnes A. N., Baasandavga U., Davaasuren A., Gonchigoo B., Gray G. C. (2020). Knowledge and practices surrounding zoonotic disease among Mongolian herding households. Pastoralism 10:8. doi: 10.1186/s13570-020-00162-5 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

