Transcriptome analysis of heat resistance regulated by quorum sensing system in Glaesserella parasuis
- PMID: 36033895
- PMCID: PMC9403865
- DOI: 10.3389/fmicb.2022.968460
Transcriptome analysis of heat resistance regulated by quorum sensing system in Glaesserella parasuis
Abstract
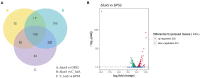
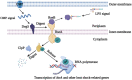
The ability of bacteria to resist heat shock allows them to adapt to different environments. In addition, heat shock resistance is known for their virulence. Our previous study showed that the AI-2/luxS quorum sensing system affects the growth characteristics, biofilm formation, and virulence of Glaesserella parasuis. The resistance of quorum sensing system deficient G. parasuis to heat shock was obviously weaker than that of wild type strain. However, the regulatory mechanism of this phenotype remains unclear. To illustrate the regulatory mechanism by which the quorum sensing system provides resistance to heat shock, the transcriptomes of wild type (GPS2), ΔluxS, and luxS complemented (C-luxS) strains were analyzed. Four hundred forty-four differentially expressed genes were identified in quorum sensing system deficient G. parasuis, which participated in multiple regulatory pathways. Furthermore, we found that G. parasuis regulates the expression of rseA, rpoE, rseB, degS, clpP, and htrA genes to resist heat shock via the quorum sensing system. We further confirmed that rseA and rpoE genes exerted an opposite regulatory effect on heat shock resistance. In conclusion, the findings of this study provide a novel insight into how the quorum sensing system affects the transcriptome of G. parasuis and regulates its heat shock resistance property.
Keywords: Glaesserella parasuis; heat shock resistance; molecular mechanism; quorum sensing; transcriptome.
Copyright © 2022 Zhang, Jiang, Cao, Zeng, Ren, Hu, Li and He.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources

