Detection and quantitation of hepatitis B surface antigen (HBsAg) on dried blood spots: a solution for easy access for hepatitis B diagnosis and elimination in remote areas
- PMID: 36034040
- PMCID: PMC9379421
- DOI: 10.11604/pamj.2022.42.100.30531
Detection and quantitation of hepatitis B surface antigen (HBsAg) on dried blood spots: a solution for easy access for hepatitis B diagnosis and elimination in remote areas
Abstract
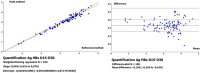
Hepatitis B virus (HBV) is generally endemic in resource-limited countries, which are characterized by a deficit of technical facilities that could delay diagnosis and treatment. To facilitate the accessibility to diagnostic and connection to treatment, evaluation, and promotion of alternatives and/or simplified strategies and inexpensive tools such as dried blood specimens need to be investigated and implemented. This study aimed to evaluate dried blood spots (DBS) for the detection and quantification of HBsAg. This study included 100 DBS from subjects tested positive for HBsAg, and 50 DBSs from subjects tested negative for HBsAg by the automate Architect i1000sr (Abbott Diagnostics, Ireland). Hepatitis B surface antigen detection was performed with determine HBsAg Alere® tests (Alere International Limited, Ireland) and Architect® HBsAg Qualitative II Assays (Abbott, Diagnostics, Ireland) after 15 and 30 days (D15, D30). For HBsAg-positive subjects, the quantification of HBsAg was performed at day zero (D0) from plasma and at D15 and D30 from the DBSs. At D15, the sensitivity and specificity were 96% and 100% for the Determine® tests and 100% and 100% for the Architect® tests, respectively. At D30, the sensitivity and specificity were 96% and 100% for the Determine® tests and 100% and 100% for the Architect® tests, respectively. For HBsAg quantification, the agreement rates were 96%, 96% and 100% between D0-D15, D0-D30 and D15-D30, respectively. This work showed that DBSs can be very useful for HBsAg detection and quantification and therefore in the management of HBV infection in resource-limited settings.
Keywords: HBsAg quantification; Hepatitis B virus; dried blood spots.
Copyright: Anna Julienne Selbé Ndiaye et al.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- World Health Organisation Hepatitis B. 2020. Accessed July 3, 2021.
-
- Lemoine M, Eholie S, Lacombe K. Reducing the neglected burden of viral hepatitis in Africa: strategies for a global approach. J Hepatol. 2015;62(2):469–476. - PubMed
-
- Kramvis A, Kew MC. Epidemiology of hepatitis B virus in Africa, its genotypes and clinical associations of genotypes. Hepatol Res. 2007;37(s1):S9–S19. - PubMed
-
- Howell J, Lemoine M, Thursz M. Prevention of materno-foetal transmission of hepatitis B in sub-Saharan Africa: the evidence, current practice and future challenges. J Viral Hepat. 2014;21(6):381–396. - PubMed
-
- Lo G, Diawara PS, Diouf NN, Faye B, Seck MC, Sow K, et al. Prévalence de l'antigène de surface du virus de l'héaptite B (Ag HBs) chez les femmes enceintes au laboratoire de l'hôpital militaire de Ouakam (HMO), Dakar. Méd Afr Noire. 2012;59(5):241–244.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
