Harmine mitigates cisplatin-induced renal injury in male mice through antioxidant, anti-inflammatory, and anti-apoptosis effects
- PMID: 36034080
- PMCID: PMC9400464
- DOI: 10.4103/1735-5362.350242
Harmine mitigates cisplatin-induced renal injury in male mice through antioxidant, anti-inflammatory, and anti-apoptosis effects
Abstract
Background and purpose: Cisplatin is a chemotherapeutic drug used to treat cancer, however, causes kidney toxicity. Harmine is a plant-derived alkaloid with a wide range of therapeutic applications. The effects of harmine on the renal side effects of cisplatin in mice were studied in this study.
Experimental approach: Forty-eight male BALB/c mice were randomly divided into eight groups (n = 6). They were treated with saline, cisplatin (5.5 mg/kg), harmine (5, 10, and 15 mg/kg/day), cisplatin + harmine (5, 10, and 15 mg/kg/day), respectively. All administrations were done daily and intraperitoneally for 4 days. The criteria related to histology, oxidation, anti-oxidation, inflammation, and apoptosis of renal tissue were evaluated.
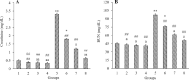
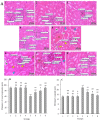
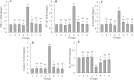
Findings / results: There was a significant decrease in total antioxidant capacity of renal tissue, renal corpuscles diameter, and IL-10 expression level in the cisplatin group than in the control group, while the values of these parameters were significantly similar to the control group in the moderate or high doses of harmine + cisplatin groups. There were significant increases in serum urea and creatinine levels, bowman space, the amounts of malondialdehyde, apoptosis rate, and TNF-α, NF-κB, IL-1β, and caspase-3 gene expressions in kidney tissue of the cisplatin group compared to the control group, while these criteria did not differ in the moderate or high doses of harmine + cisplatin groups.
Conclusion and implications: Harmine protected the kidneys against cisplatin-induced damage. Antioxidant, anti-inflammatory, and anti-apoptotic harmine properties were involved in this healing effect.
Keywords: Antioxidants; Harmine; Oxidative stress; Toxicity.
Copyright: © 2022 Research in Pharmaceutical Sciences.
Conflict of interest statement
The authors declared no conflict of interest in this study.
Figures
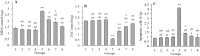
References
-
- Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73((9)):994–1007. DOI: 10.1038/sj.ki.5002786. - PubMed
-
- Manohar S, Leung N. Cisplatin nephrotoxicity: a review of the literature. J Nephrol. 2018;31(1):15–25. DOI: 10.1007/s40620-017-0392-z. - PubMed
-
- Santos NA, Catao CS, Martins NM, Curti C, Bianchi MLP, Santos AC. Cisplatin-induced nephrotoxicity is associated with oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Arch Toxicol. 2007;81(7):495–504. DOI: 10.1007/s00204-006-0173-2. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
