Genetic and clinical features of cerebellar ataxia with RFC1 biallelic repeat expansions in Japan
- PMID: 36034314
- PMCID: PMC9404689
- DOI: 10.3389/fneur.2022.952493
Genetic and clinical features of cerebellar ataxia with RFC1 biallelic repeat expansions in Japan
Abstract
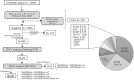
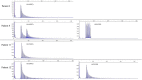
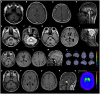
The recessive intronic pentanucleotide repeat AAGGG expansion of replication factor complex subunit 1 (RFC1) is associated with cerebellar ataxia, sensory neuropathy, and vestibular areflexia syndrome. And the clinical spectrum has been continuously expanding. We conducted this study to demonstrate the clinical and genetic features of a large-scale case series of Japanese patients with cerebellar ataxia with RFC1 repeat expansions. We examined 1,289 Japanese patients with cerebellar ataxia and analyzed RFC1 repeat expansions in 840 patients, excluding those with genetic diagnoses or an autosomal dominant inheritance pattern. For individuals where no product was obtained by flanking polymerase chain reaction (PCR), repeat-primed PCR was performed using primers specific for the following four repeat motifs: AAAAG, AAAGG, AAGGG, and ACAGG. RFC1 analysis revealed multitype biallelic pathogenic repeat expansions in 15 patients, including (AAGGG)exp/(AAGGG)exp in seven patients, (ACAGG)exp/(ACAGG)exp in three patients, (AAGGG)exp/(ACAGG)exp in four patients, and (AAGGG)exp/(AAAGG)15(AAGGG)exp in one patient. Clinical analysis showed various combinations of cerebellar ataxia, vestibular dysfunction, neuropathy, cognitive decline, autonomic dysfunction, chronic cough, pyramidal tract disorder, parkinsonism, involuntary movement, and muscle fasciculation. Pathological RFC1 repeat expansions account for 1.8% (15/840) of undiagnosed patients with cerebellar ataxia and sporadic/recessive/unclassified inheritance. Screening of RFC1 repeat expansions should be considered in patients with cerebellar ataxia, irrespective of their subtype and onset age.
Keywords: (AAAGG)10−25(AAGGG)exp; (AAGGG)exp; (ACAGG)exp; RFC1; cerebellar ataxia.
Copyright © 2022 Ando, Higuchi, Yuan, Yoshimura, Higashi, Takeuchi, Hobara, Kojima, Noguchi, Takei, Hiramatsu, Nozuma, Sakiyama, Hashiguchi, Matsuura, Okamoto, Nagai and Takashima.
Figures
References
LinkOut - more resources
Full Text Sources

