Conversion of calcium-l-methylfolate and (6S)-5-methyltetrahydrofolic acid glucosamine salt into dietary folate equivalents
- PMID: 36034319
- PMCID: PMC9399872
- DOI: 10.2903/j.efsa.2022.7452
Conversion of calcium-l-methylfolate and (6S)-5-methyltetrahydrofolic acid glucosamine salt into dietary folate equivalents
Abstract
Following a request from the European Commission, the EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) was asked to deliver a scientific opinion on the conversion of calcium-l-methylfolate and (6S)-5-methyltetrahydrofolic acid glucosamine salt (collectively called 5-MTHF hereafter) into dietary folate equivalents (DFE). Following a systematic review, the conclusions of the opinion are based on one intervention study in adults for intakes < 400 μg/day and three intervention studies in adults for intakes ≥ 400 μg/day. At intakes below 400 μg/day, folic acid (FA) is assumed to be linearly related to responses of biomarkers of intake and status and is an appropriate comparator for deriving a DFE conversion factor for 5-MTHF. It is proposed to use the same factor as for folic acid for conversion of 5-MTHF into DFE for intakes < 400 μg/day. As such intake levels are unlikely to be exceeded through fortified food consumption, the conversion factor of 1.7 relative to natural food folate (NF) could be applied to 5-MTHF added to foods and to food supplements providing < 400 μg/day. At 400 μg/day, 5-MTHF was found to be more bioavailable than folic acid and a conversion factor of 2 is proposed for this intake level and for higher intakes. The derived DFE equations are DFE = NF + 1.7 × FA + 1.7 × 5-MTHF for fortified foods and food supplements providing intakes < 400 μg/day; and DFE = NF + 1.7 × FA + 2.0 × 5-MTHF for food supplements providing intakes ≥ 400 μg/day. Although this assessment applies to calcium-L-methylfolate and 5-MTHF glucosamine salt, it is considered that the influence of the cation on bioavailability is likely to be within the margin of error of the proposed DFE equations. Therefore, the proposed equations can also be applied to 5-MTHF associated with other cations.
Keywords: 5‐MTHF glucosamine; CaLMF; DFE; bioavailability; food for specific groups; food supplements; fortified food.
© 2022 Wiley‐VCH Verlag GmbH & Co. KgaA on behalf of the European Food Safety Authority.
Figures
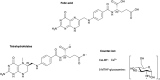
glucosamine5‐MTHF‐glucosamine: (6S)‐5‐methyltetrahydrofolic acid glucosamine salt, Ca: calcium.

DMG: dimethylglycine, DNA: deoxyribonucleic acid, FAD: flavin adenine dinucleotide, MTHFR: methylene tetrahydrofolate reductase, PLP: pyroxidal 5′‐phosphate, THF: tetrahydrofolate.

The red dashed line corresponds to a ratio equal to one (i.e. similar effect of 5‐MTHF and folic acid on RBC folate concentrations). The size of the point is proportional to the weight of the study as defined by the inverse of its variance in the meta‐regression model.
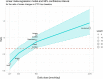
The red dashed line corresponds to a ratio equal to one (i.e. similar effect of 5‐MTHF and folic acid on PTF concentrations). The size of the point is proportional to the weight of the study as defined by the inverse of its variance in the meta‐regression model.

Similar articles
-
Safety of monosodium salt of l-5-methyltetrahydrofolic acid as a novel food pursuant to Regulation (EU) 2015/2283 and the bioavailability of folate from this source in the context of Directive 2002/46/EC, Regulation (EU) No 609/2013 and Regulation (EC) No 1925/2006.EFSA J. 2023 Nov 29;21(11):e8417. doi: 10.2903/j.efsa.2023.8417. eCollection 2023 Nov. EFSA J. 2023. PMID: 38035146 Free PMC article.
-
Scientific opinion on the tolerable upper intake level for folate.EFSA J. 2023 Nov 13;21(11):e08353. doi: 10.2903/j.efsa.2023.8353. eCollection 2023 Nov. EFSA J. 2023. PMID: 37965303 Free PMC article.
-
Enhanced oral bioavailability of a novel folate salt: comparison with folic acid and a calcium folate salt in a pharmacokinetic study in rats.Minerva Ginecol. 2016 Apr;68(2):99-105. Minerva Ginecol. 2016. PMID: 27008238
-
Folate, folic acid and 5-methyltetrahydrofolate are not the same thing.Xenobiotica. 2014 May;44(5):480-8. doi: 10.3109/00498254.2013.845705. Epub 2014 Feb 4. Xenobiotica. 2014. PMID: 24494987 Review.
-
Nutritional challenges for older adults in Europe: current status and future directions.Proc Nutr Soc. 2019 May;78(2):221-233. doi: 10.1017/S0029665118002744. Epub 2019 Jan 30. Proc Nutr Soc. 2019. PMID: 30696516 Review.
Cited by
-
Safety of monosodium salt of l-5-methyltetrahydrofolic acid as a novel food pursuant to Regulation (EU) 2015/2283 and the bioavailability of folate from this source in the context of Directive 2002/46/EC, Regulation (EU) No 609/2013 and Regulation (EC) No 1925/2006.EFSA J. 2023 Nov 29;21(11):e8417. doi: 10.2903/j.efsa.2023.8417. eCollection 2023 Nov. EFSA J. 2023. PMID: 38035146 Free PMC article.
-
Biological, dietetic and pharmacological properties of vitamin B9.NPJ Sci Food. 2025 Mar 13;9(1):30. doi: 10.1038/s41538-025-00396-w. NPJ Sci Food. 2025. PMID: 40075081 Free PMC article. Review.
-
Guidance on scientific principles and data requirements for the safety and relative bioavailability assessment of new micronutrient sources.EFSA J. 2024 Sep 30;22(9):e8946. doi: 10.2903/j.efsa.2024.8946. eCollection 2024 Sep. EFSA J. 2024. PMID: 39351444 Free PMC article.
-
Scientific opinion on the tolerable upper intake level for folate.EFSA J. 2023 Nov 13;21(11):e08353. doi: 10.2903/j.efsa.2023.8353. eCollection 2023 Nov. EFSA J. 2023. PMID: 37965303 Free PMC article.
-
The Bioavailability of Drugs-The Current State of Knowledge.Molecules. 2023 Dec 11;28(24):8038. doi: 10.3390/molecules28248038. Molecules. 2023. PMID: 38138529 Free PMC article. Review.
References
-
- Akoglu B, Schrott M, Bolouri H, Jaffari A, Kutschera E, Caspary WF and Faust D, 2008. The folic acid metabolite L‐5‐methyltetrahydrofolate effectively reduces total serum homocysteine level in orthotopic liver transplant recipients: a double‐blind placebo‐controlled study. European Journal of Clinical Nutrition, 62, 796–801. 10.1038/sj.ejcn.1602778 - DOI - PubMed
-
- Alegría A, Garcia‐Llatas G and Cilla A, 2015. Static digestion models: general introduction. In: Verhoeckx K, Cotter P, López‐Expósito I, Kleiveland C, Lea T, Mackie A, Requena T, Swiatecka D and Wichers H (eds.). The impact of food bioactives on health. Cham, Heidelberg, New York, Dordrecht, London, Springer. pp. 3–12. - PubMed
-
- Arkbåge K, Verwei M, Havenaar R and Witthöft C, 2003. Bioaccessibility of folic acid and (6S)‐5‐methyltetrahydrofolate decreases after the addition of folate‐binding protein to yogurt as studied in a dynamic in vitro gastrointestinal model. Journal of Nutrition, 133, 3678–3683. 10.1093/jn/133.11.3678 - DOI - PubMed
-
- Bailey SW and Ayling JE, 2009. The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. Proceedings of the National Academy of Sciences of the United States of America, 106, 15424–15429. 10.1073/pnas.0902072106 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
