INSPIRE: Safety and tolerability of inhaled Yutrepia (treprostinil) in pulmonary arterial hypertension (PAH)
- PMID: 36034402
- PMCID: PMC9400582
- DOI: 10.1002/pul2.12119
INSPIRE: Safety and tolerability of inhaled Yutrepia (treprostinil) in pulmonary arterial hypertension (PAH)
Abstract
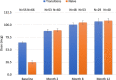
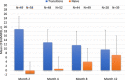
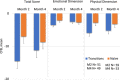
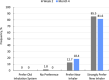
The INSPIRE trial was a Phase 3, open-label, multicenter trial (LTI-301) that enrolled patients with pulmonary arterial hypertension (PAH) ≥ 18 years of age who transitioned to Yutrepia from nebulized treprostinil (Transition) or added Yutrepia to prostacyclin naïve patients on ≤2 nonprostacyclin oral therapies. The objectives of the trial were to evaluate the safety and tolerability of Yutrepia (dry-powder formulation of treprostinil) in patients with PAH. The primary safety measures were the incidence of adverse events (AEs) and serious AEs. Exploratory efficacy measures were also assessed during the trial. Transition patients initiated Yutrepia at a dose comparable to their nebulized treprostinil dose while prostacyclin naïve patients received 26.5-mcg QID; up-titration in 26.5-mcg increments was permitted for both groups. A total of 121 patients were enrolled, of which 29 patients discontinued from the trial, with the most common reason being AEs. Eighty percent of the Transition group and 96% of the prostacyclin naïve group titrated to a dose ≥79.5 mcg QID at Day 360, respectively, with one patient achieving a dose of 212-mcg QID. The most common AEs were cough, headache, upper respiratory tract infection, dyspnea, dizziness, throat irritation, diarrhea, chest discomfort, fatigue, and nasopharyngitis. Most of these events were considered treatment-related though mild to moderate in severity and expected for prostacyclin therapy administered by inhalation. In an evaluation of exploratory efficacy measures, patients remained stable or improved over the 1 year of treatment. Yutrepia was found to be a convenient, safe, and well-tolerated inhaled prostacyclin treatment option for PAH patients.
Keywords: combination therapy; dry‐powder inhaler; prostacyclin; pulmonary arterial hypertension; treprostinil.
© 2022 The Authors. Pulmonary Circulation published by John Wiley & Sons Ltd on behalf of Pulmonary Vascular Research Institute.
Conflict of interest statement
N. S. H.: Liquidia and Aerovate, consulting fees; Altavant, lectures, presentations, speakers bureaus, manuscript writing, and/or educational events; Liquidia, expert testimony; Liquidia, support for attending meetings and/or travel; Merck and United Therapeutics, data safety monitoring board or advisory board. J. P. F.: Liquidia, advisory board. S. S.: ACCP CHEST and United Therapeutics, research grants; Liquidia, steering committee; Gossamer Bio, United Therapeutics, and Bayer, advisor; Bayer, United Therapeutics, and Johnson & Johnson, speaker; Johnson & Johnson, planned patent; Bayer, advisory board; GSK, endpoint adjudication committee. R. L. B.: Bayer, Abbot, United Therapeutics, Abbott, Gossamer Bio, and Acceleron, consulting fees; Society of Heart and Lung Transplantation and the Pulmonary Vascular Disease Institute, leadership and/or fiduciary role. I. R. P.: Janssen, United Therapeutics, PhaseBio, and Tenax, research grants and/or contracts; Altavant, Janssen, United Therapeutics, and Liquidia, consulting fees; Medscape and Med on the Go, lectures, presentations, speakers bureaus, manuscript writing and/or educational events; Bayer, expert testimony; Altavant, Janssen, United Therapeutics, and Liquidia, data safety monitoring or advisory board; ISHLT, leadership or fiduciary role. D. B.: Acceleron, Arena/United Therapeutics, Altavant, Actelion/Janssen/Johnson & Johnson, Ikaria, Reata, Complexa Liquidia, and Merck, research grants; Acceleron, Altavant, Bayer, Merck, and Liquidia, consulting fees; Practice Point Communications, lectures, presentations, speakers bureaus, manuscript writing and/or educational events; Bayer, expert testimony; United Therapeutics, data safety or advisory board; USPHSR (United States Pulmonary Hypertension Scientific Registry), leadership or fiduciary role; Johnson & Johnson, stock or stock options. R. P. F.: NHLBI, research grant; Up to Date, royalties or licenses; Janssen, Liquidia, Shouti, Altavant, Gossamer Bio, Bayer, and Tenax, consulting fees; United Therapeutics, France Foundation, and Janssen, lectures, presentations, speakers bureaus, manuscript writing and/or educational events; Janssen and Liquidia, data safety or advisory board; Tenax, stock or stock options. S. P.: Liquidia, employee, stock or stock options. A. G.: Liquidia, employee, attending meetings and/or travel patents, and stock or stock options. T. M. B.: Bayer, research grant; Liquidia and Bayer, steering committee and consulting fees; NHLBI and MESA, data safety and/or advisory board.
Figures

References
-
- Gomberg‐Maitland M, Olschewski H. Prostacyclin therapies for the treatment of pulmonary arterial hypertension. Eur Respir J. 2008;31(4):891–901. - PubMed
-
- Remodulin (treprostinil injection) [package insert] . Research Triangle Park, North Carolina: United Therapeutics Corp.; 2018.
-
- Veletri (epoprostenol) [package insert] . South San Francisco, California: Actelion Pharmaceuticals US Inc.; 2018.
LinkOut - more resources
Full Text Sources

