Cdc42 upregulation under high glucose induces podocyte apoptosis and impairs β-cell insulin secretion
- PMID: 36034435
- PMCID: PMC9399854
- DOI: 10.3389/fendo.2022.905703
Cdc42 upregulation under high glucose induces podocyte apoptosis and impairs β-cell insulin secretion
Abstract
Objectives: The progressive impairment of β-cell function results in prolonged deterioration in patients with type 2 diabetes mellitus (T2DM). Interestingly, the finding on pancreatitis secondary to renal injury suggests that potential communication exists between kidney and pancreas. Therefore, we aimed to investigate cell division cycle 42 (Cdc42)-mediated podocyte apoptosis and its effect on insulin secretion in islet β-cells.
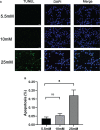
Methods: Type 2 diabetic nephropathy mouse models were established to identify the expression of Cdc42 in podocytes by immunohistochemistry. An in vitro co-culture of mouse podocyte MPC5 and β-TC6 cells was preliminarily established. Subsequently, podocyte apoptosis induced by high glucose and Cdc42 was detected by TUNEL staining and western blotting. In addition, the JNK pathway was examined to determine the mechanism of apoptosis in MPC5 cells. Finally, insulin secretion and expression in β-TC6 cells as well as malondialdehyde (MDA) and superoxide dismutase (SOD) levels in both cell types were examined after the regulation of Cdc42 in MPC5 cells.
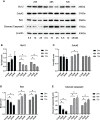
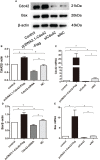
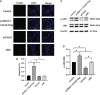
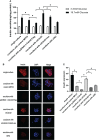
Results: Cdc42 was highly expressed in the podocytes of diabetic nephropathy mice. Exposure to 25 mM glucose for 48 h induced a significant upregulation of Cdc42, Bax, and cleaved caspase-3 as well as a decreased Bcl-2 expression. In addition, marked apoptosis of MPC5 cells was observed compared to normal glucose treatment. After transfection with Cdc42 plasmid, apoptosis of MPC5 cells was enhanced with an increased expression of p-JNK, whereas inhibition of Cdc42 significantly alleviated podocyte apoptosis accompanied by a downregulation of p-JNK. The glucose-stimulated insulin secretion level of β-TC6 cells decreased after the upregulation of Cdc42 in MPC5 cells. Immunofluorescence staining for insulin showed that co-culture with MPC5 cells carrying the Cdc42 plasmid significantly reduced insulin expression, whereas inhibition of Cdc42 in MPC5 cells alleviated the above-mentioned abnormality of β-TC6 cells. The expression of Cdc42 and p-p38 in β-TC6 cells increased following the upregulation of Cdc42 in MPC5 cells; this was concurrent with augmented MDA levels and decreased SOD activity. The opposite result was observed for Cdc42 knockdown in MPC5 cells.
Conclusions: Cdc42 in podocytes plays a crucial role in insulin secretion by β-cells, which may provide a new therapeutic target to prevent the vicious cycle of β-cell dysfunction in T2DM.
Keywords: Cdc42; apoptosis; co-culture; podocyte; β-cells.
Copyright © 2022 Jiang, Xu, Yao, Zhang, Li, Zhang, Xie, Xing, Zhang, Zhou, Liao and Dong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Van Geertruyden J, Toussaint C. [Acute pancreatitis after renal transplantation]. Acta Chir Belg (1967) 66(3):271–80. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

