Targeting of the intracellular redox balance by metal complexes towards anticancer therapy
- PMID: 36034648
- PMCID: PMC9405673
- DOI: 10.3389/fchem.2022.967337
Targeting of the intracellular redox balance by metal complexes towards anticancer therapy
Abstract
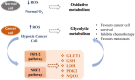
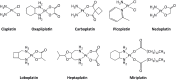
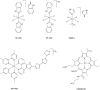
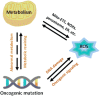
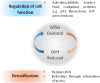
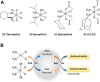
The development of cancers is often linked to the alteration of essential redox processes, and therefore, oxidoreductases involved in such mechanisms can be considered as attractive molecular targets for the development of new therapeutic strategies. On the other hand, for more than two decades, transition metals derivatives have been leading the research on drugs as alternatives to platinum-based treatments. The success of such compounds is particularly due to their attractive redox kinetics properties, favorable oxidation states, as well as routes of action different to interactions with DNA, in which redox interactions are crucial. For instance, the activity of oxidoreductases such as PHD2 (prolyl hydroxylase domain-containing protein) which can regulate angiogenesis in tumors, LDH (lactate dehydrogenase) related to glycolysis, and enzymes, such as catalases, SOD (superoxide dismutase), TRX (thioredoxin) or GSH (glutathione) involved in controlling oxidative stress, can be altered by metal effectors. In this review, we wish to discuss recent results on how transition metal complexes have been rationally designed to impact on redox processes, in search for effective and more specific cancer treatments.
Keywords: anticancer therapy; oxidoreductases; redox balance; transition metals; tumor metabolism.
Copyright © 2022 Murillo, Gaiddon and Le Lagadec.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Agonigi G., Riedel T., Gay M. P., Biancalana L., Oñate E., Dyson P. J., et al. (2016). Arene osmium complexes with ethacrynic acid-modified ligands: Synthesis, characterization, and evaluation of intracellular glutathione S-transferase inhibition and antiproliferative activity. Organometallics 35, 1046–1056. 10.1021/acs.organomet.6b00197 10.1021/acs.organomet.6b00197 | Google Scholar - DOI - DOI
-
- Alessio E., Messori L. (2019). NAMI-A and KP1019/1339, two iconic ruthenium anticancer drug candidates face-to-face: A case story in medicinal inorganic chemistry. Molecules 24, 1995. 10.3390/molecules24101995 10.3390/molecules24101995 | Google Scholar - DOI - DOI - PMC - PubMed
-
- Allen J. F., Alexciev K., Håkansson G. (1995). Photosynthesis: Regulation by redox signalling. Curr. Biol. 5, 869–872. 10.1016/S0960-9822(95)00176-X PubMed Abstract | 10.1016/S0960-9822(95)00176-X | Google Scholar - DOI - DOI - PubMed
-
- Allocati N., Masulli M., Ilio C. D., Federici L. (2018). Glutathione transferases: Substrates, inihibitors and pro-drugs in cancer and neurodegenerative diseases. Oncogenesis 7, 8. 10.1038/s41389-017-0025-3 PubMed Abstract | 10.1038/s41389-017-0025-3 | Google Scholar - DOI - DOI - PMC - PubMed
-
- Alpeeva I. S., Soukharev V. S., Alexandrova L., Shilova N. V., Bovin N. V., Csoregi E., et al. (2003). Cyclometalated ruthenium(II) complexes as efficient redox mediators in peroxidase catalysis. J. Biol. Inorg. Chem. 8, 683–688. 10.1007/s00775-003-0467-2 PubMed Abstract | 10.1007/s00775-003-0467-2 | Google Scholar - DOI - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

