Damage associated molecular patterns and neutrophil extracellular traps in acute pancreatitis
- PMID: 36034701
- PMCID: PMC9411527
- DOI: 10.3389/fcimb.2022.927193
Damage associated molecular patterns and neutrophil extracellular traps in acute pancreatitis
Abstract
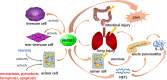
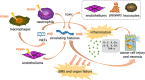
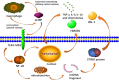
Previous researches have emphasized a trypsin-centered theory of acute pancreatitis (AP) for more than a century. With additional studies into the pathogenesis of AP, new mechanisms have been explored. Among them, the role of immune response bears great importance. Pro-inflammatory substances, especially damage-associated molecular patterns (DAMPs), play an essential role in activating, signaling, and steering inflammation. Meanwhile, activated neutrophils attach great importance to the immune defense by forming neutrophil extracellular traps (NETs), which cause ductal obstruction, premature trypsinogen activation, and modulate inflammation. In this review, we discuss the latest advances in understanding the pathological role of DAMPs and NETs in AP and shed light on the flexible crosstalk between these vital inflammatory mediators. We, then highlight the potentially promising treatment for AP targeting DAMPs and NETs, with a focus on novel insights into the mechanism, diagnosis, and management of AP.
Keywords: DAMPs (damage-associated molecular patterns); HMGB1 (high mobility group box 1); HSP (heat shock protein); NETs (neutrophil extracellular traps); acute pancreatitis (AP); histone.
Copyright © 2022 Zhou, Jin, Pan, Lin, Yang, Ambe, Basharat, Zimmer, Wang and Hong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

