Neutrophils incite and macrophages avert electrical storm after myocardial infarction
- PMID: 36034743
- PMCID: PMC9410341
- DOI: 10.1038/s44161-022-00094-w
Neutrophils incite and macrophages avert electrical storm after myocardial infarction
Abstract
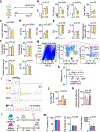
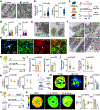
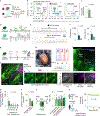
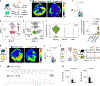
Sudden cardiac death, arising from abnormal electrical conduction, occurs frequently in patients with coronary heart disease. Myocardial ischemia simultaneously induces arrhythmia and massive myocardial leukocyte changes. In this study, we optimized a mouse model in which hypokalemia combined with myocardial infarction triggered spontaneous ventricular tachycardia in ambulatory mice, and we showed that major leukocyte subsets have opposing effects on cardiac conduction. Neutrophils increased ventricular tachycardia via lipocalin-2 in mice, whereas neutrophilia associated with ventricular tachycardia in patients. In contrast, macrophages protected against arrhythmia. Depleting recruited macrophages in Ccr2 -/- mice or all macrophage subsets with Csf1 receptor inhibition increased both ventricular tachycardia and fibrillation. Higher arrhythmia burden and mortality in Cd36 -/- and Mertk -/- mice, viewed together with reduced mitochondrial integrity and accelerated cardiomyocyte death in the absence of macrophages, indicated that receptor-mediated phagocytosis protects against lethal electrical storm. Thus, modulation of leukocyte function provides a potential therapeutic pathway for reducing the risk of sudden cardiac death.
Conflict of interest statement
Competing interests J.G. and M.N. filed an invention disclosure on the STORM model. M.N. has received funds or material research support from Alnylam, Biotronik, CSL Behring, GlycoMimetics, GlaxoSmithKline, Medtronic, Novartis and Pfizer as well as consulting fees from Eli Lilly, Biogen, Gimv, IFM Therapeutics, Molecular Imaging, Sigilon and Verseau Therapeutics. P.T.E. has received sponsored research support from Bayer AG and IBM Health and has consulted for Bayer AG, Novartis and MyoKardia. A.D.A. has received sponsored research support from Amgen and Philip Research. The other authors declare no competing interests.
Figures









Comment in
-
Macrophages and neutrophils modulate arrhythmia risk after myocardial infarction.Nat Rev Cardiol. 2022 Sep;19(9):573. doi: 10.1038/s41569-022-00758-x. Nat Rev Cardiol. 2022. PMID: 35879543 No abstract available.
References
-
- Chugh SS et al. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J. Am. Coll. Cardiol. 44, 1268–1275 (2004). - PubMed
-
- Borne RT, Varosy PD & Masoudi FA Implantable cardioverter-defibrillator shocks: epidemiology, outcomes, and therapeutic approaches. JAMA Intern. Med. 173, 859–865 (2013). - PubMed
-
- Suga H Ventricular energetics. Physiol. Rev. 70, 247–277 (1990). - PubMed
Grants and funding
- R01 HL149647/HL/NHLBI NIH HHS/United States
- DP2 AR075321/AR/NIAMS NIH HHS/United States
- P30 DK043351/DK/NIDDK NIH HHS/United States
- R01 HL092577/HL/NHLBI NIH HHS/United States
- R35 HL135752/HL/NHLBI NIH HHS/United States
- FS/SCRF/20/32005/BHF_/British Heart Foundation/United Kingdom
- CH/16/1/32013/BHF_/British Heart Foundation/United Kingdom
- K24 HL105780/HL/NHLBI NIH HHS/United States
- R01 HL125428/HL/NHLBI NIH HHS/United States
- FS/TF/20/33001/BHF_/British Heart Foundation/United Kingdom
- PG/2019/34842/BHF_/British Heart Foundation/United Kingdom
- P01 HL142494/HL/NHLBI NIH HHS/United States
- R00 HL129168/HL/NHLBI NIH HHS/United States
- R01 HL144515/HL/NHLBI NIH HHS/United States
- P30 DK057521/DK/NIDDK NIH HHS/United States
- R35 HL139598/HL/NHLBI NIH HHS/United States
- R01 HL155097/HL/NHLBI NIH HHS/United States
- R21 EB026762/EB/NIBIB NIH HHS/United States
- FS/16/7/31843/BHF_/British Heart Foundation/United Kingdom
- F32 HL158040/HL/NHLBI NIH HHS/United States
- P30 DK135043/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
