Licensing of Orphan Medicinal Products-Use of Real-World Data and Other External Data on Efficacy Aspects in Marketing Authorization Applications Concluded at the European Medicines Agency Between 2019 and 2021
- PMID: 36034814
- PMCID: PMC9413272
- DOI: 10.3389/fphar.2022.920336
Licensing of Orphan Medicinal Products-Use of Real-World Data and Other External Data on Efficacy Aspects in Marketing Authorization Applications Concluded at the European Medicines Agency Between 2019 and 2021
Abstract
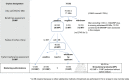
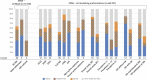
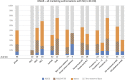
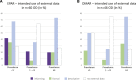
Background: Reference to so-called real-world data is more often made in marketing authorization applications for medicines intended to diagnose, prevent or treat rare diseases compared to more common diseases. We provide granularity on the type and aim of any external data on efficacy aspects from both real-world data sources and external trial data as discussed in regulatory submissions of orphan designated medicinal products in the EU. By quantifying the contribution of external data according to various regulatory characteristics, we aimed at identifying specific opportunities for external data in the field of orphan conditions. Methods: Information on external data in regulatory documents covering 72 orphan designations was extracted. Our sample comprised public assessment reports for approved, refused, or withdrawn applications concluded from 2019-2021 at the European Medicines Agency. Products with an active orphan designation at the time of submission were scrutinized regarding the role of external data on efficacy aspects in the context of marketing authorization applications, or on the criterion of "significant benefit" for the confirmation of the orphan designation at the time of licensing. The reports allowed a broad distinction between clinical development, regulatory decision making, and intended post-approval data collection. We defined three categories of external data, administrative data, structured clinical data, and external trial data (from clinical trials not sponsored by the applicant), and noted whether external data concerned the therapeutic context of the disease or the product under review. Results: While reference to external data with respect to efficacy aspects was included in 63% of the approved medicinal products in the field of rare diseases, 37% of marketing authorization applications were exclusively based on the dedicated clinical development plan for the product under review. Purely administrative data did not play any role in our sample of reports, but clinical data collected in a structured manner (from routine care or clinical research) were often used to inform on the trial design. Two additional recurrent themes for the use of external data were the contextualization of results, especially to confirm the orphan designation at the time of licensing, and reassurance of a large difference in treatment effect size or consistency of effects observed in clinical trials and practice. External data on the product under review were restricted to either active substances already belonging to the standard of care even before authorization or to compassionate use schemes. Furthermore, external data were considered pivotal for marketing authorization only exceptionally and only for active substances already in use within the specific therapeutic indication. Applications for the rarest conditions and those without authorized treatment alternatives were especially prominent with respect to the use of external data from real-world data sources both in the pre- and post-approval setting. Conclusion: Specific opportunities for external data in the setting of marketing authorizations in the field of rare diseases were identified. Ongoing initiatives of fostering systematic data collection are promising steps for a more efficient medicinal product development in the field of rare diseases.
Keywords: drug development; efficacy; marketing authorization application; orphan drugs; orphan medicinal product; real-world data.
Copyright © 2022 Naumann-Winter, Wolter, Hermes, Malikova, Lilienthal, Meier, Kalland and Magrelli.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor VSB declared a shared committee Committee for Orphan Medicinal Products of the EMA with the authors FNW, EM, MEK, AM at the time of review.
Figures

References
-
- Big Data Steering Group (BDSG) (2021). Report (EMA/40400/2022). Available at: https://www.ema.europa.eu/en/documents/report/big-data-steering-group-bd... (Accessed April 14, 2022).
Publication types
LinkOut - more resources
Full Text Sources

